Kia Khezeli
Center for Individualized Medicine, Department of Surgery, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA
Few-Shot LoRA Adaptation of a Flow-Matching Foundation Model for Cross-Spectral Object Detection
Jan 07, 2026Abstract:Foundation models for vision are predominantly trained on RGB data, while many safety-critical applications rely on non-visible modalities such as infrared (IR) and synthetic aperture radar (SAR). We study whether a single flow-matching foundation model pre-trained primarily on RGB images can be repurposed as a cross-spectral translator using only a few co-measured examples, and whether the resulting synthetic data can enhance downstream detection. Starting from FLUX.1 Kontext, we insert low-rank adaptation (LoRA) modules and fine-tune them on just 100 paired images per domain for two settings: RGB to IR on the KAIST dataset and RGB to SAR on the M4-SAR dataset. The adapted model translates RGB images into pixel-aligned IR/SAR, enabling us to reuse existing bounding boxes and train object detection models purely in the target modality. Across a grid of LoRA hyperparameters, we find that LPIPS computed on only 50 held-out pairs is a strong proxy for downstream performance: lower LPIPS consistently predicts higher mAP for YOLOv11n on both IR and SAR, and for DETR on KAIST IR test data. Using the best LPIPS-selected LoRA adapter, synthetic IR from external RGB datasets (LLVIP, FLIR ADAS) improves KAIST IR pedestrian detection, and synthetic SAR significantly boosts infrastructure detection on M4-SAR when combined with limited real SAR. Our results suggest that few-shot LoRA adaptation of flow-matching foundation models is a promising path toward foundation-style support for non-visible modalities.
CausalARC: Abstract Reasoning with Causal World Models
Sep 03, 2025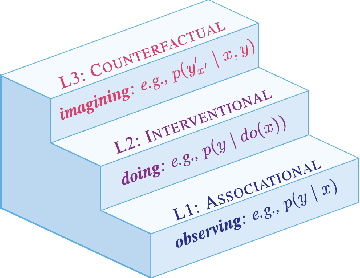
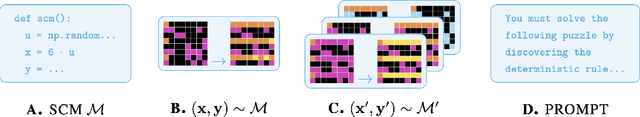

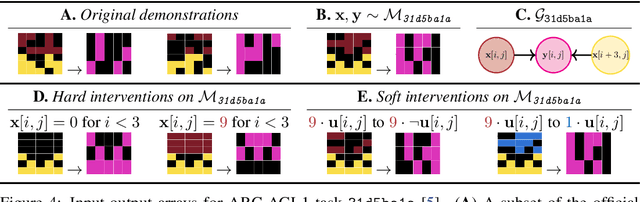
Abstract:Reasoning requires adaptation to novel problem settings under limited data and distribution shift. This work introduces CausalARC: an experimental testbed for AI reasoning in low-data and out-of-distribution regimes, modeled after the Abstraction and Reasoning Corpus (ARC). Each CausalARC reasoning task is sampled from a fully specified causal world model, formally expressed as a structural causal model. Principled data augmentations provide observational, interventional, and counterfactual feedback about the world model in the form of few-shot, in-context learning demonstrations. As a proof-of-concept, we illustrate the use of CausalARC for four language model evaluation settings: (1) abstract reasoning with test-time training, (2) counterfactual reasoning with in-context learning, (3) program synthesis, and (4) causal discovery with logical reasoning.
A multi-cohort study on prediction of acute brain dysfunction states using selective state space models
Mar 11, 2024

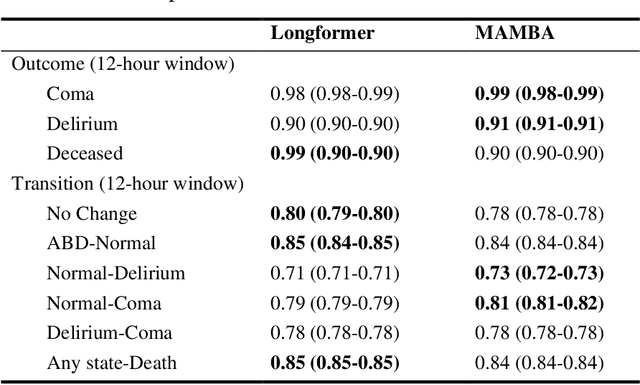

Abstract:Assessing acute brain dysfunction (ABD), including delirium and coma in the intensive care unit (ICU), is a critical challenge due to its prevalence and severe implications for patient outcomes. Current diagnostic methods rely on infrequent clinical observations, which can only determine a patient's ABD status after onset. Our research attempts to solve these problems by harnessing Electronic Health Records (EHR) data to develop automated methods for ABD prediction for patients in the ICU. Existing models solely predict a single state (e.g., either delirium or coma), require at least 24 hours of observation data to make predictions, do not dynamically predict fluctuating ABD conditions during ICU stay (typically a one-time prediction), and use small sample size, proprietary single-hospital datasets. Our research fills these gaps in the existing literature by dynamically predicting delirium, coma, and mortality for 12-hour intervals throughout an ICU stay and validating on two public datasets. Our research also introduces the concept of dynamically predicting critical transitions from non-ABD to ABD and between different ABD states in real time, which could be clinically more informative for the hospital staff. We compared the predictive performance of two state-of-the-art neural network models, the MAMBA selective state space model and the Longformer Transformer model. Using the MAMBA model, we achieved a mean area under the receiving operator characteristic curve (AUROC) of 0.95 on outcome prediction of ABD for 12-hour intervals. The model achieves a mean AUROC of 0.79 when predicting transitions between ABD states. Our study uses a curated dataset from the University of Florida Health Shands Hospital for internal validation and two publicly available datasets, MIMIC-IV and eICU, for external validation, demonstrating robustness across ICU stays from 203 hospitals and 140,945 patients.
APRICOT: Acuity Prediction in Intensive Care Unit (ICU): Predicting Stability, Transitions, and Life-Sustaining Therapies
Nov 03, 2023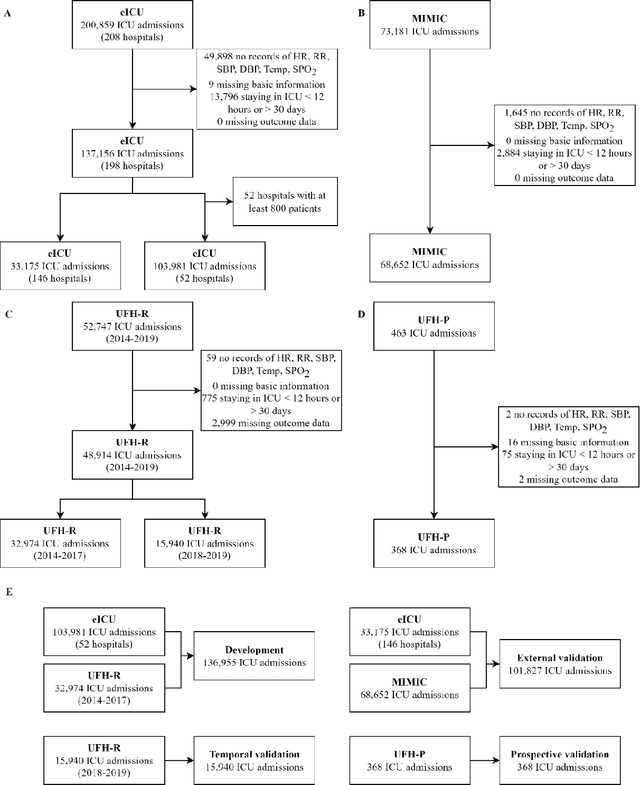

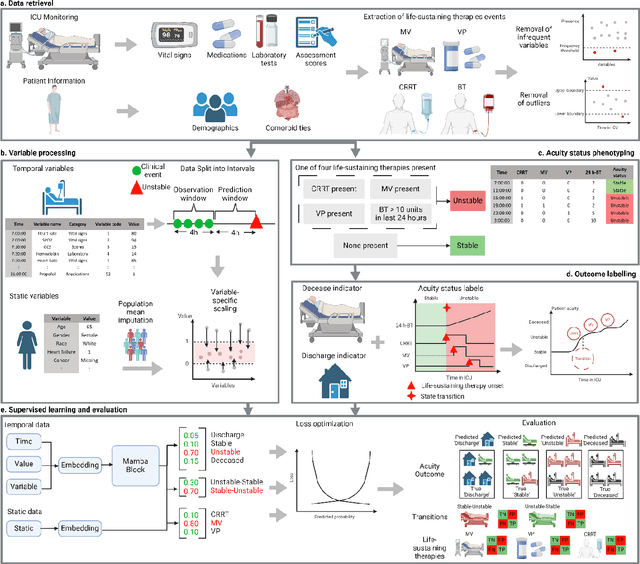
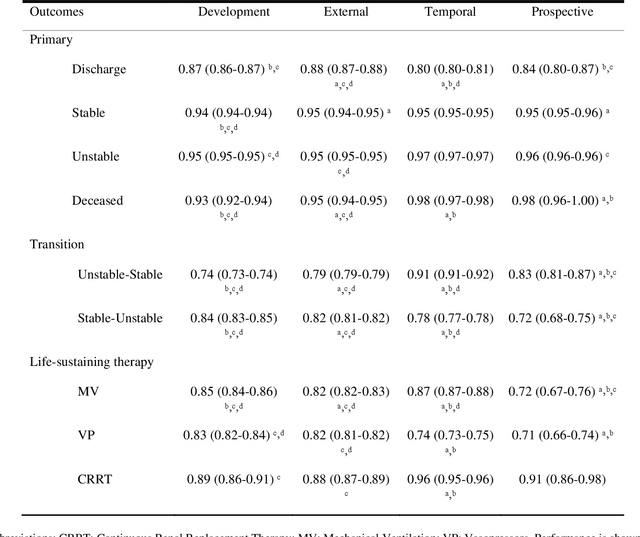
Abstract:The acuity state of patients in the intensive care unit (ICU) can quickly change from stable to unstable, sometimes leading to life-threatening conditions. Early detection of deteriorating conditions can result in providing more timely interventions and improved survival rates. Current approaches rely on manual daily assessments. Some data-driven approaches have been developed, that use mortality as a proxy of acuity in the ICU. However, these methods do not integrate acuity states to determine the stability of a patient or the need for life-sustaining therapies. In this study, we propose APRICOT (Acuity Prediction in Intensive Care Unit), a Transformer-based neural network to predict acuity state in real-time in ICU patients. We develop and extensively validate externally, temporally, and prospectively the APRICOT model on three large datasets: University of Florida Health (UFH), eICU Collaborative Research Database (eICU), and Medical Information Mart for Intensive Care (MIMIC)-IV. The performance of APRICOT shows comparable results to state-of-the-art mortality prediction models (external AUROC 0.93-0.93, temporal AUROC 0.96-0.98, and prospective AUROC 0.98) as well as acuity prediction models (external AUROC 0.80-0.81, temporal AUROC 0.77-0.78, and prospective AUROC 0.87). Furthermore, APRICOT can make predictions for the need for life-sustaining therapies, showing comparable results to state-of-the-art ventilation prediction models (external AUROC 0.80-0.81, temporal AUROC 0.87-0.88, and prospective AUROC 0.85), and vasopressor prediction models (external AUROC 0.82-0.83, temporal AUROC 0.73-0.75, prospective AUROC 0.87). This tool allows for real-time acuity monitoring of a patient and can provide helpful information to clinicians to make timely interventions. Furthermore, the model can suggest life-sustaining therapies that the patient might need in the next hours in the ICU.
Transformers in Healthcare: A Survey
Jun 30, 2023



Abstract:With Artificial Intelligence (AI) increasingly permeating various aspects of society, including healthcare, the adoption of the Transformers neural network architecture is rapidly changing many applications. Transformer is a type of deep learning architecture initially developed to solve general-purpose Natural Language Processing (NLP) tasks and has subsequently been adapted in many fields, including healthcare. In this survey paper, we provide an overview of how this architecture has been adopted to analyze various forms of data, including medical imaging, structured and unstructured Electronic Health Records (EHR), social media, physiological signals, and biomolecular sequences. Those models could help in clinical diagnosis, report generation, data reconstruction, and drug/protein synthesis. We identified relevant studies using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We also discuss the benefits and limitations of using transformers in healthcare and examine issues such as computational cost, model interpretability, fairness, alignment with human values, ethical implications, and environmental impact.
Transformer Models for Acute Brain Dysfunction Prediction
Mar 13, 2023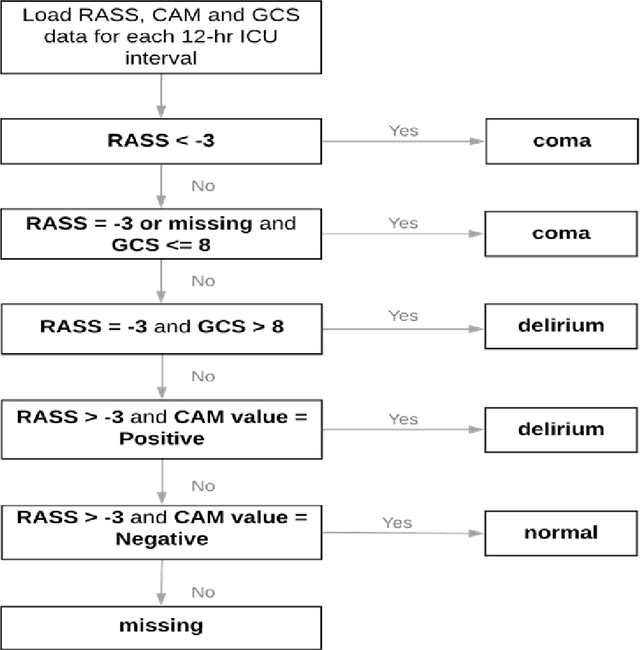
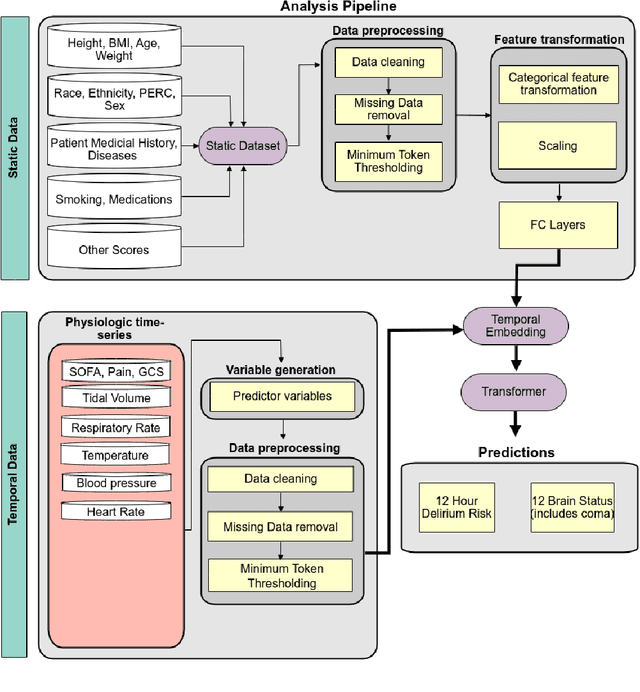

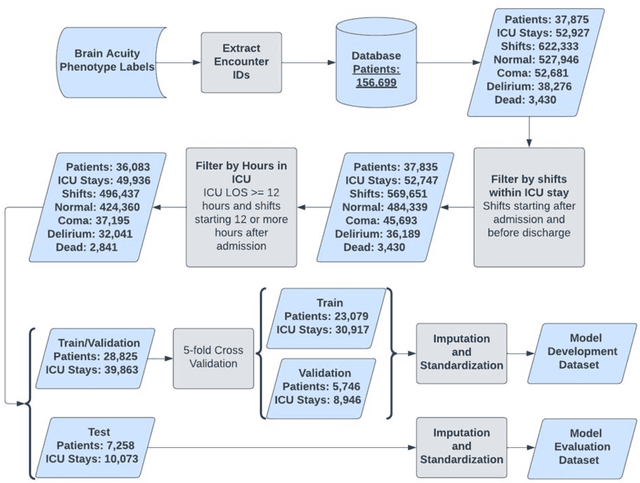
Abstract:Acute brain dysfunctions (ABD), which include coma and delirium, are prevalent in the ICU, especially among older patients. The current approach in manual assessment of ABD by care providers may be sporadic and subjective. Hence, there exists a need for a data-driven robust system automating the assessment and prediction of ABD. In this work, we develop a machine learning system for real-time prediction of ADB using Electronic Health Record (HER) data. Our data processing pipeline enables integration of static and temporal data, and extraction of features relevant to ABD. We train several state-of-the-art transformer models and baseline machine learning models including CatBoost and XGB on the data that was collected from patients admitted to the ICU at UF Shands Hospital. We demonstrate the efficacy of our system for tasks related to acute brain dysfunction including binary classification of brain acuity and multi-class classification (i.e., coma, delirium, death, or normal), achieving a mean AUROC of 0.953 on our Long-former implementation. Our system can then be deployed for real-time prediction of ADB in ICUs to reduce the number of incidents caused by ABD. Moreover, the real-time system has the potential to reduce costs, duration of patients stays in the ICU, and mortality among those afflicted.
Predicting risk of delirium from ambient noise and light information in the ICU
Mar 11, 2023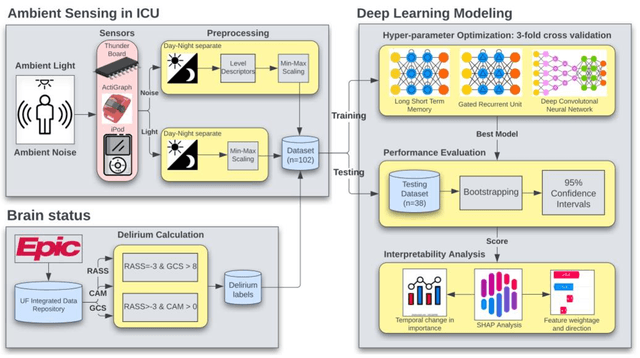
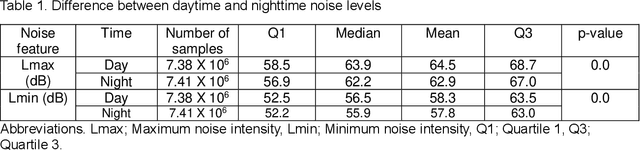
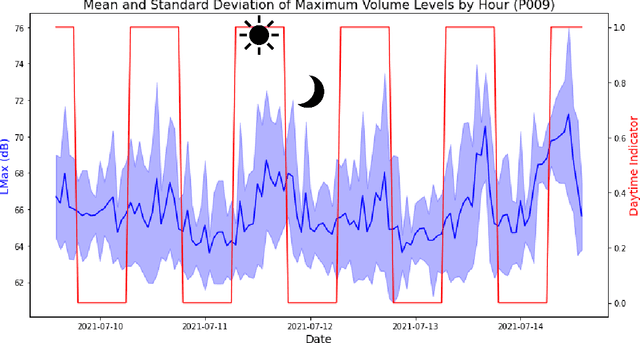
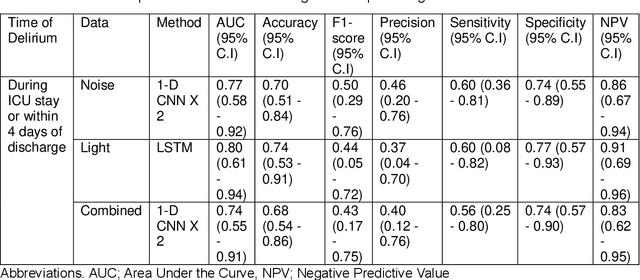
Abstract:Existing Intensive Care Unit (ICU) delirium prediction models do not consider environmental factors despite strong evidence of their influence on delirium. This study reports the first deep-learning based delirium prediction model for ICU patients using only ambient noise and light information. Ambient light and noise intensities were measured from ICU rooms of 102 patients from May 2021 to September 2022 using Thunderboard, ActiGraph sensors and an iPod with AudioTools application. These measurements were divided into daytime (0700 to 1859) and nighttime (1900 to 0659). Deep learning models were trained using this data to predict the incidence of delirium during ICU stay or within 4 days of discharge. Finally, outcome scores were analyzed to evaluate the importance and directionality of every feature. Daytime noise levels were significantly higher than nighttime noise levels. When using only noise features or a combination of noise and light features 1-D convolutional neural networks (CNN) achieved the strongest performance: AUC=0.77, 0.74; Sensitivity=0.60, 0.56; Specificity=0.74, 0.74; Precision=0.46, 0.40 respectively. Using only light features, Long Short-Term Memory (LSTM) networks performed best: AUC=0.80, Sensitivity=0.60, Specificity=0.77, Precision=0.37. Maximum nighttime and minimum daytime noise levels were the strongest positive and negative predictors of delirium respectively. Nighttime light level was a stronger predictor of delirium than daytime light level. Total influence of light features outweighed that of noise features on the second and fourth day of ICU stay. This study shows that ambient light and noise intensities are strong predictors of long-term delirium incidence in the ICU. It reveals that daytime and nighttime environmental factors might influence delirium differently and that the importance of light and noise levels vary over the course of an ICU stay.
AI-Enhanced Intensive Care Unit: Revolutionizing Patient Care with Pervasive Sensing
Mar 11, 2023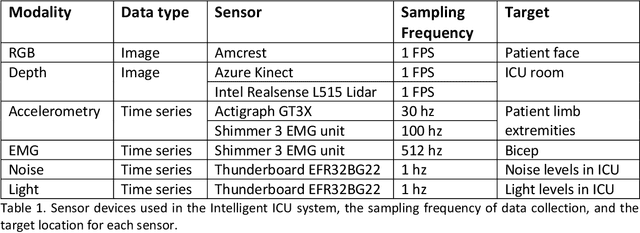
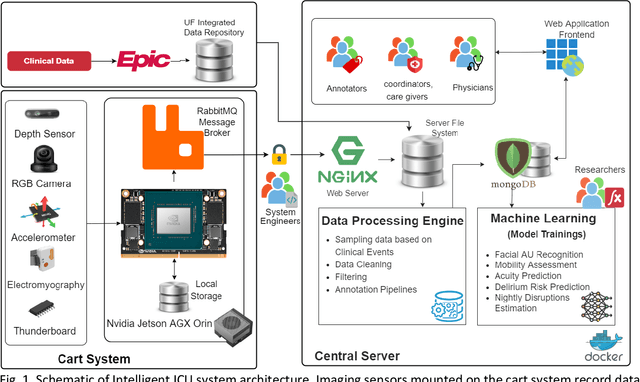
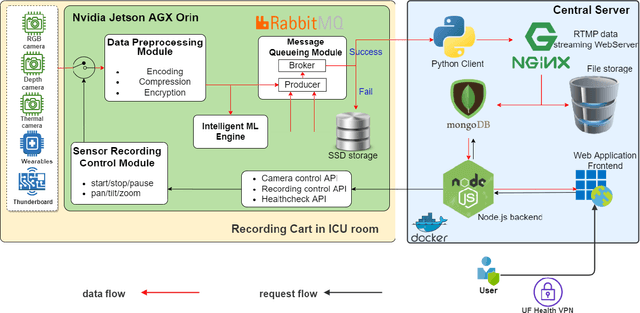
Abstract:The intensive care unit (ICU) is a specialized hospital space where critically ill patients receive intensive care and monitoring. Comprehensive monitoring is imperative in assessing patients conditions, in particular acuity, and ultimately the quality of care. However, the extent of patient monitoring in the ICU is limited due to time constraints and the workload on healthcare providers. Currently, visual assessments for acuity, including fine details such as facial expressions, posture, and mobility, are sporadically captured, or not captured at all. These manual observations are subjective to the individual, prone to documentation errors, and overburden care providers with the additional workload. Artificial Intelligence (AI) enabled systems has the potential to augment the patient visual monitoring and assessment due to their exceptional learning capabilities. Such systems require robust annotated data to train. To this end, we have developed pervasive sensing and data processing system which collects data from multiple modalities depth images, color RGB images, accelerometry, electromyography, sound pressure, and light levels in ICU for developing intelligent monitoring systems for continuous and granular acuity, delirium risk, pain, and mobility assessment. This paper presents the Intelligent Intensive Care Unit (I2CU) system architecture we developed for real-time patient monitoring and visual assessment.
End-to-End Machine Learning Framework for Facial AU Detection in Intensive Care Units
Nov 12, 2022



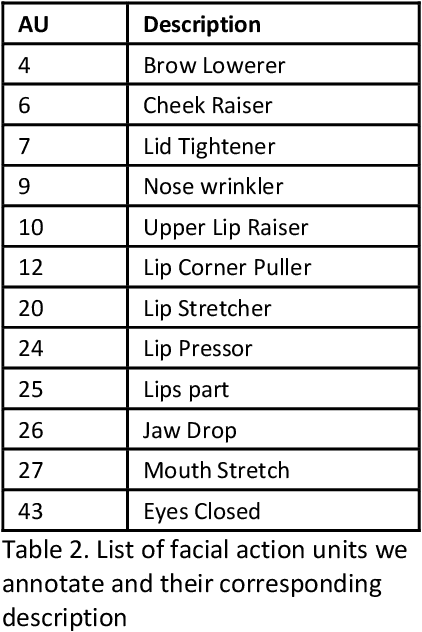
Abstract:Pain is a common occurrence among patients admitted to Intensive Care Units. Pain assessment in ICU patients still remains a challenge for clinicians and ICU staff, specifically in cases of non-verbal sedated, mechanically ventilated, and intubated patients. Current manual observation-based pain assessment tools are limited by the frequency of pain observations administered and are subjective to the observer. Facial behavior is a major component in observation-based tools. Furthermore, previous literature shows the feasibility of painful facial expression detection using facial action units (AUs). However, these approaches are limited to controlled or semi-controlled environments and have never been validated in clinical settings. In this study, we present our Pain-ICU dataset, the largest dataset available targeting facial behavior analysis in the dynamic ICU environment. Our dataset comprises 76,388 patient facial image frames annotated with AUs obtained from 49 adult patients admitted to ICUs at the University of Florida Health Shands hospital. In this work, we evaluated two vision transformer models, namely ViT and SWIN, for AU detection on our Pain-ICU dataset and also external datasets. We developed a completely end-to-end AU detection pipeline with the objective of performing real-time AU detection in the ICU. The SWIN transformer Base variant achieved 0.88 F1-score and 0.85 accuracy on the held-out test partition of the Pain-ICU dataset.
Beyond Low Earth Orbit: Biomonitoring, Artificial Intelligence, and Precision Space Health
Dec 22, 2021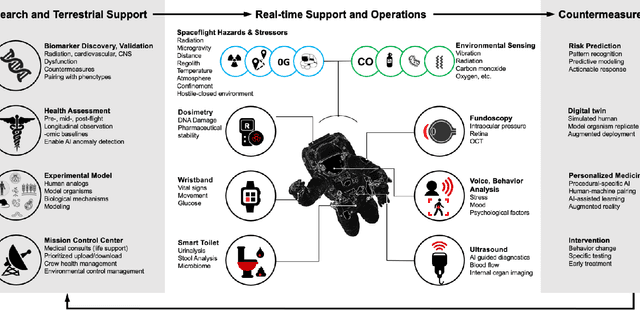
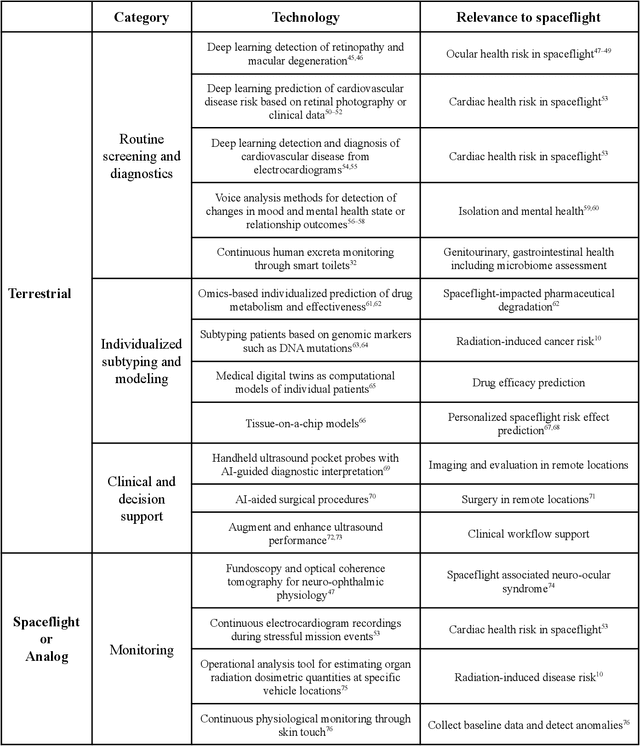
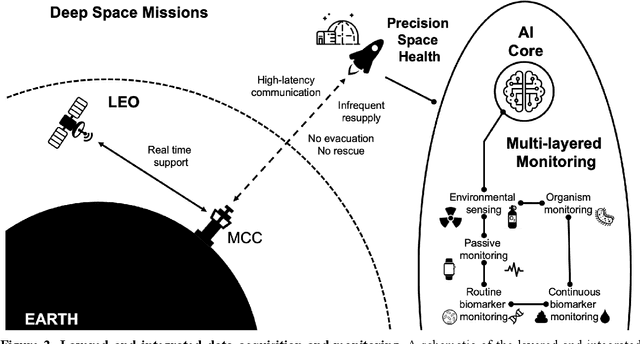
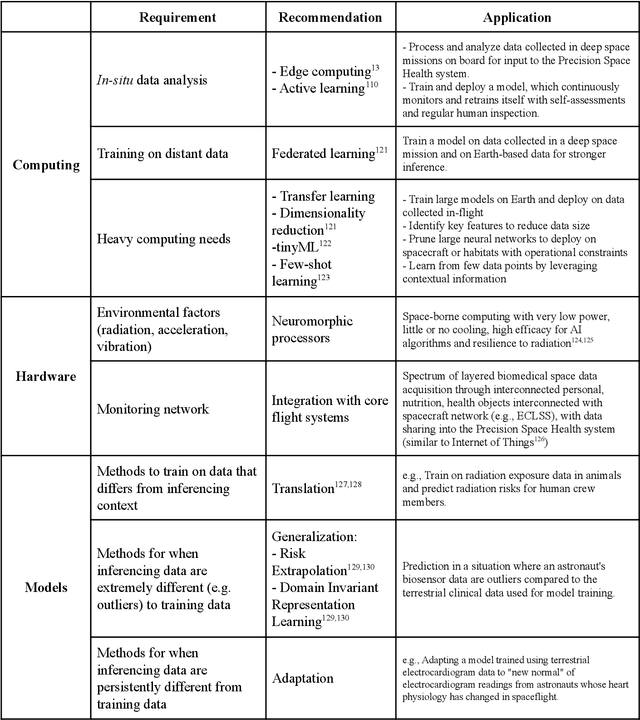
Abstract:Human space exploration beyond low Earth orbit will involve missions of significant distance and duration. To effectively mitigate myriad space health hazards, paradigm shifts in data and space health systems are necessary to enable Earth-independence, rather than Earth-reliance. Promising developments in the fields of artificial intelligence and machine learning for biology and health can address these needs. We propose an appropriately autonomous and intelligent Precision Space Health system that will monitor, aggregate, and assess biomedical statuses; analyze and predict personalized adverse health outcomes; adapt and respond to newly accumulated data; and provide preventive, actionable, and timely insights to individual deep space crew members and iterative decision support to their crew medical officer. Here we present a summary of recommendations from a workshop organized by the National Aeronautics and Space Administration, on future applications of artificial intelligence in space biology and health. In the next decade, biomonitoring technology, biomarker science, spacecraft hardware, intelligent software, and streamlined data management must mature and be woven together into a Precision Space Health system to enable humanity to thrive in deep space.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge