Sabyasachi Bandyopadhyay
MANGO: Multimodal Acuity traNsformer for intelliGent ICU Outcomes
Dec 13, 2024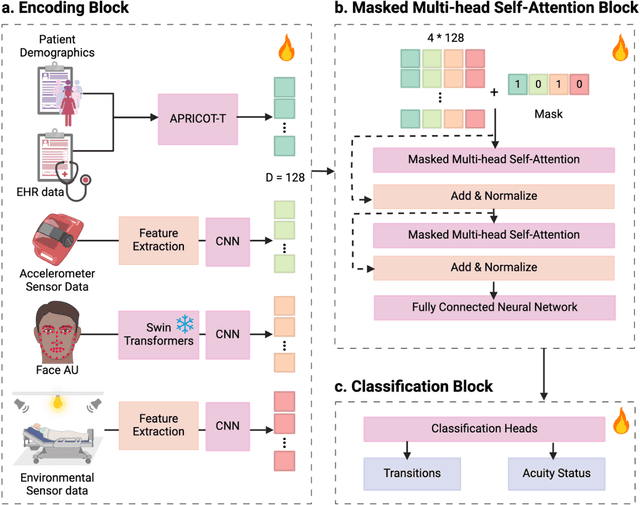
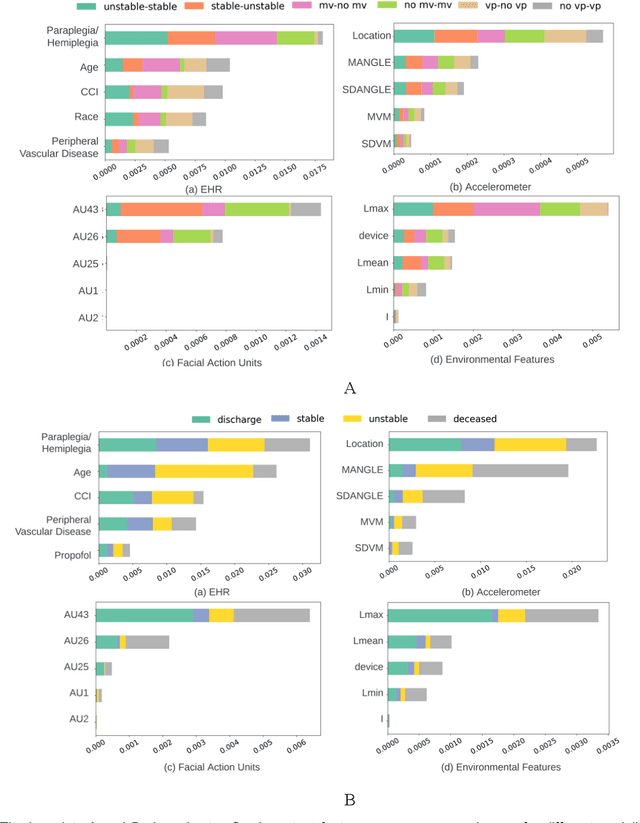
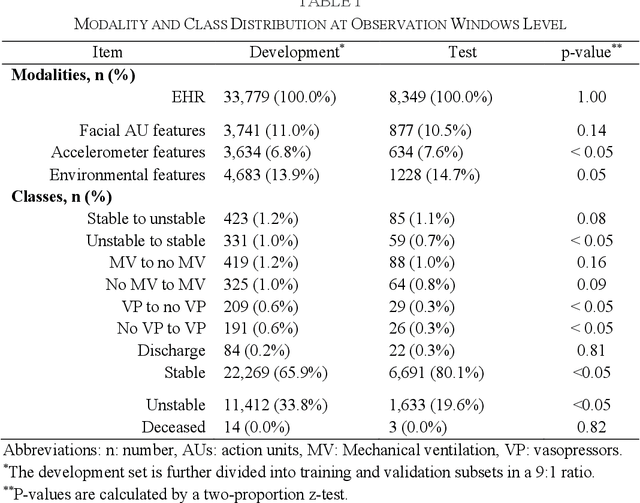
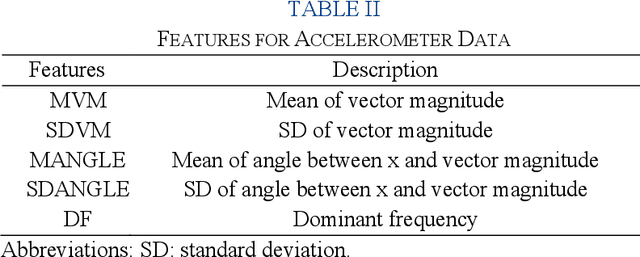
Abstract:Estimation of patient acuity in the Intensive Care Unit (ICU) is vital to ensure timely and appropriate interventions. Advances in artificial intelligence (AI) technologies have significantly improved the accuracy of acuity predictions. However, prior studies using machine learning for acuity prediction have predominantly relied on electronic health records (EHR) data, often overlooking other critical aspects of ICU stay, such as patient mobility, environmental factors, and facial cues indicating pain or agitation. To address this gap, we present MANGO: the Multimodal Acuity traNsformer for intelliGent ICU Outcomes, designed to enhance the prediction of patient acuity states, transitions, and the need for life-sustaining therapy. We collected a multimodal dataset ICU-Multimodal, incorporating four key modalities, EHR data, wearable sensor data, video of patient's facial cues, and ambient sensor data, which we utilized to train MANGO. The MANGO model employs a multimodal feature fusion network powered by Transformer masked self-attention method, enabling it to capture and learn complex interactions across these diverse data modalities even when some modalities are absent. Our results demonstrated that integrating multiple modalities significantly improved the model's ability to predict acuity status, transitions, and the need for life-sustaining therapy. The best-performing models achieved an area under the receiver operating characteristic curve (AUROC) of 0.76 (95% CI: 0.72-0.79) for predicting transitions in acuity status and the need for life-sustaining therapy, while 0.82 (95% CI: 0.69-0.89) for acuity status prediction...
Peri-AIIMS: Perioperative Artificial Intelligence Driven Integrated Modeling of Surgeries using Anesthetic, Physical and Cognitive Statuses for Predicting Hospital Outcomes
Oct 29, 2024



Abstract:The association between preoperative cognitive status and surgical outcomes is a critical, yet scarcely explored area of research. Linking intraoperative data with postoperative outcomes is a promising and low-cost way of evaluating long-term impacts of surgical interventions. In this study, we evaluated how preoperative cognitive status as measured by the clock drawing test contributed to predicting length of hospital stay, hospital charges, average pain experienced during follow-up, and 1-year mortality over and above intraoperative variables, demographics, preoperative physical status and comorbidities. We expanded our analysis to 6 specific surgical groups where sufficient data was available for cross-validation. The clock drawing images were represented by 10 constructional features discovered by a semi-supervised deep learning algorithm, previously validated to differentiate between dementia and non-dementia patients. Different machine learning models were trained to classify postoperative outcomes in hold-out test sets. The models were compared to their relative performance, time complexity, and interpretability. Shapley Additive Explanations (SHAP) analysis was used to find the most predictive features for classifying different outcomes in different surgical contexts. Relative classification performances achieved by different feature sets showed that the perioperative cognitive dataset which included clock drawing features in addition to intraoperative variables, demographics, and comorbidities served as the best dataset for 12 of 18 possible surgery-outcome combinations...
A multi-cohort study on prediction of acute brain dysfunction states using selective state space models
Mar 11, 2024

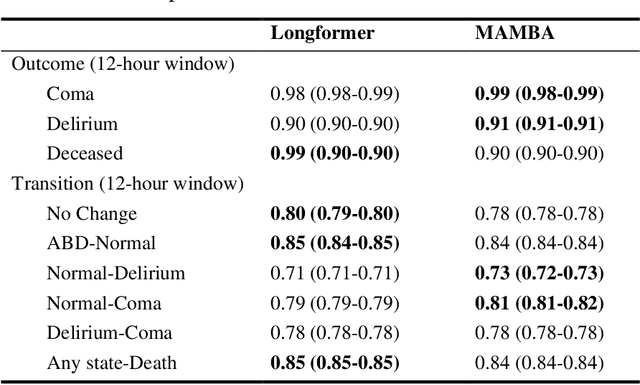

Abstract:Assessing acute brain dysfunction (ABD), including delirium and coma in the intensive care unit (ICU), is a critical challenge due to its prevalence and severe implications for patient outcomes. Current diagnostic methods rely on infrequent clinical observations, which can only determine a patient's ABD status after onset. Our research attempts to solve these problems by harnessing Electronic Health Records (EHR) data to develop automated methods for ABD prediction for patients in the ICU. Existing models solely predict a single state (e.g., either delirium or coma), require at least 24 hours of observation data to make predictions, do not dynamically predict fluctuating ABD conditions during ICU stay (typically a one-time prediction), and use small sample size, proprietary single-hospital datasets. Our research fills these gaps in the existing literature by dynamically predicting delirium, coma, and mortality for 12-hour intervals throughout an ICU stay and validating on two public datasets. Our research also introduces the concept of dynamically predicting critical transitions from non-ABD to ABD and between different ABD states in real time, which could be clinically more informative for the hospital staff. We compared the predictive performance of two state-of-the-art neural network models, the MAMBA selective state space model and the Longformer Transformer model. Using the MAMBA model, we achieved a mean area under the receiving operator characteristic curve (AUROC) of 0.95 on outcome prediction of ABD for 12-hour intervals. The model achieves a mean AUROC of 0.79 when predicting transitions between ABD states. Our study uses a curated dataset from the University of Florida Health Shands Hospital for internal validation and two publicly available datasets, MIMIC-IV and eICU, for external validation, demonstrating robustness across ICU stays from 203 hospitals and 140,945 patients.
Leveraging Computer Vision in the Intensive Care Unit (ICU) for Examining Visitation and Mobility
Mar 10, 2024



Abstract:Despite the importance of closely monitoring patients in the Intensive Care Unit (ICU), many aspects are still assessed in a limited manner due to the time constraints imposed on healthcare providers. For example, although excessive visitations during rest hours can potentially exacerbate the risk of circadian rhythm disruption and delirium, it is not captured in the ICU. Likewise, while mobility can be an important indicator of recovery or deterioration in ICU patients, it is only captured sporadically or not captured at all. In the past few years, the computer vision field has found application in many domains by reducing the human burden. Using computer vision systems in the ICU can also potentially enable non-existing assessments or enhance the frequency and accuracy of existing assessments while reducing the staff workload. In this study, we leverage a state-of-the-art noninvasive computer vision system based on depth imaging to characterize ICU visitations and patients' mobility. We then examine the relationship between visitation and several patient outcomes, such as pain, acuity, and delirium. We found an association between deteriorating patient acuity and the incidence of delirium with increased visitations. In contrast, self-reported pain, reported using the Defense and Veteran Pain Rating Scale (DVPRS), was correlated with decreased visitations. Our findings highlight the feasibility and potential of using noninvasive autonomous systems to monitor ICU patients.
The Potential of Wearable Sensors for Assessing Patient Acuity in Intensive Care Unit (ICU)
Nov 03, 2023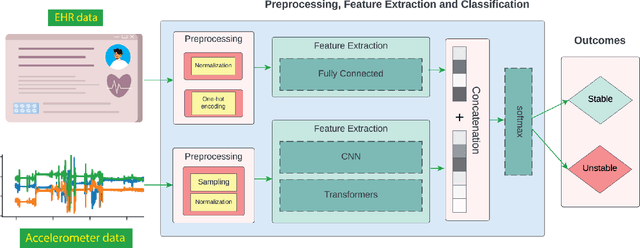
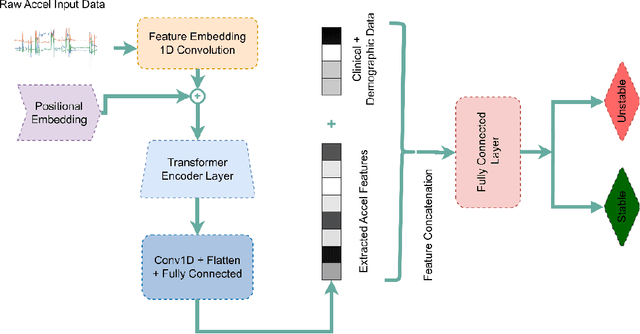

Abstract:Acuity assessments are vital in critical care settings to provide timely interventions and fair resource allocation. Traditional acuity scores rely on manual assessments and documentation of physiological states, which can be time-consuming, intermittent, and difficult to use for healthcare providers. Furthermore, such scores do not incorporate granular information such as patients' mobility level, which can indicate recovery or deterioration in the ICU. We hypothesized that existing acuity scores could be potentially improved by employing Artificial Intelligence (AI) techniques in conjunction with Electronic Health Records (EHR) and wearable sensor data. In this study, we evaluated the impact of integrating mobility data collected from wrist-worn accelerometers with clinical data obtained from EHR for developing an AI-driven acuity assessment score. Accelerometry data were collected from 86 patients wearing accelerometers on their wrists in an academic hospital setting. The data was analyzed using five deep neural network models: VGG, ResNet, MobileNet, SqueezeNet, and a custom Transformer network. These models outperformed a rule-based clinical score (SOFA= Sequential Organ Failure Assessment) used as a baseline, particularly regarding the precision, sensitivity, and F1 score. The results showed that while a model relying solely on accelerometer data achieved limited performance (AUC 0.50, Precision 0.61, and F1-score 0.68), including demographic information with the accelerometer data led to a notable enhancement in performance (AUC 0.69, Precision 0.75, and F1-score 0.67). This work shows that the combination of mobility and patient information can successfully differentiate between stable and unstable states in critically ill patients.
APRICOT: Acuity Prediction in Intensive Care Unit (ICU): Predicting Stability, Transitions, and Life-Sustaining Therapies
Nov 03, 2023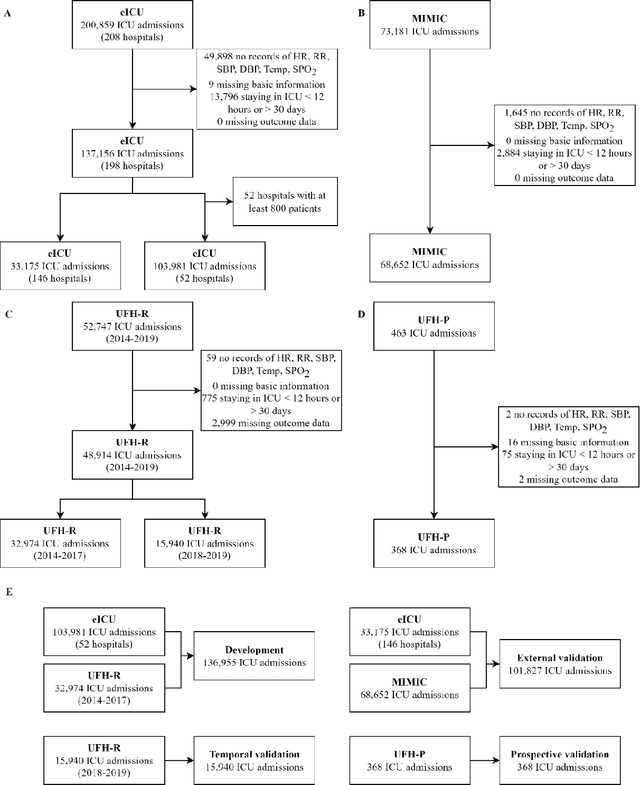

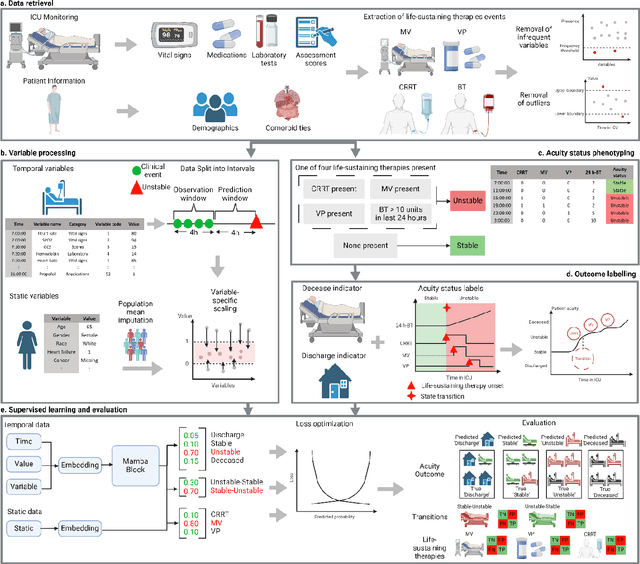
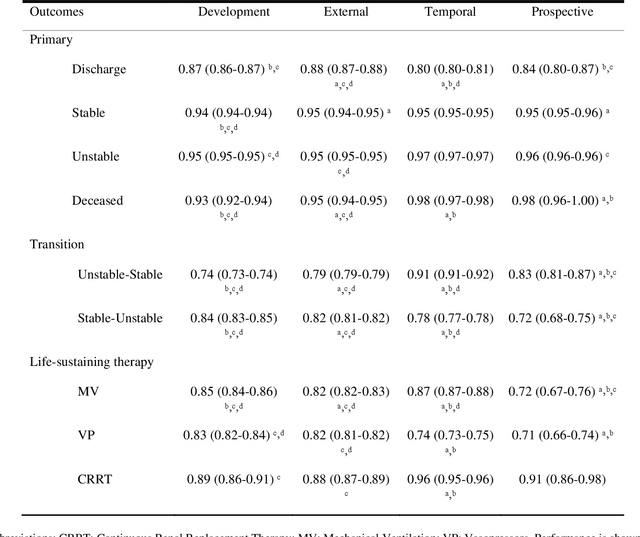
Abstract:The acuity state of patients in the intensive care unit (ICU) can quickly change from stable to unstable, sometimes leading to life-threatening conditions. Early detection of deteriorating conditions can result in providing more timely interventions and improved survival rates. Current approaches rely on manual daily assessments. Some data-driven approaches have been developed, that use mortality as a proxy of acuity in the ICU. However, these methods do not integrate acuity states to determine the stability of a patient or the need for life-sustaining therapies. In this study, we propose APRICOT (Acuity Prediction in Intensive Care Unit), a Transformer-based neural network to predict acuity state in real-time in ICU patients. We develop and extensively validate externally, temporally, and prospectively the APRICOT model on three large datasets: University of Florida Health (UFH), eICU Collaborative Research Database (eICU), and Medical Information Mart for Intensive Care (MIMIC)-IV. The performance of APRICOT shows comparable results to state-of-the-art mortality prediction models (external AUROC 0.93-0.93, temporal AUROC 0.96-0.98, and prospective AUROC 0.98) as well as acuity prediction models (external AUROC 0.80-0.81, temporal AUROC 0.77-0.78, and prospective AUROC 0.87). Furthermore, APRICOT can make predictions for the need for life-sustaining therapies, showing comparable results to state-of-the-art ventilation prediction models (external AUROC 0.80-0.81, temporal AUROC 0.87-0.88, and prospective AUROC 0.85), and vasopressor prediction models (external AUROC 0.82-0.83, temporal AUROC 0.73-0.75, prospective AUROC 0.87). This tool allows for real-time acuity monitoring of a patient and can provide helpful information to clinicians to make timely interventions. Furthermore, the model can suggest life-sustaining therapies that the patient might need in the next hours in the ICU.
Transformers in Healthcare: A Survey
Jun 30, 2023



Abstract:With Artificial Intelligence (AI) increasingly permeating various aspects of society, including healthcare, the adoption of the Transformers neural network architecture is rapidly changing many applications. Transformer is a type of deep learning architecture initially developed to solve general-purpose Natural Language Processing (NLP) tasks and has subsequently been adapted in many fields, including healthcare. In this survey paper, we provide an overview of how this architecture has been adopted to analyze various forms of data, including medical imaging, structured and unstructured Electronic Health Records (EHR), social media, physiological signals, and biomolecular sequences. Those models could help in clinical diagnosis, report generation, data reconstruction, and drug/protein synthesis. We identified relevant studies using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We also discuss the benefits and limitations of using transformers in healthcare and examine issues such as computational cost, model interpretability, fairness, alignment with human values, ethical implications, and environmental impact.
Predicting risk of delirium from ambient noise and light information in the ICU
Mar 11, 2023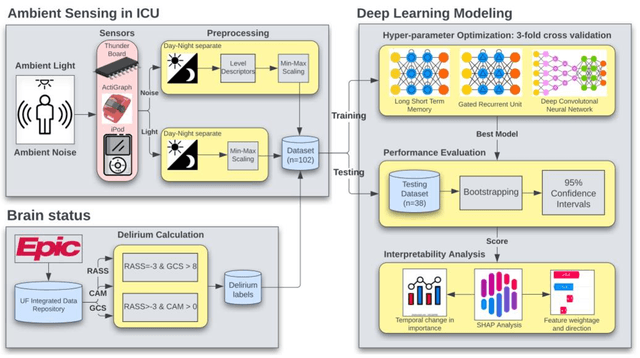
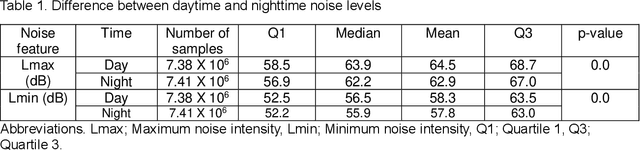
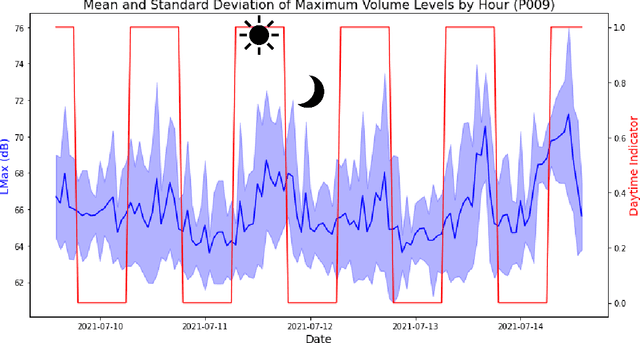
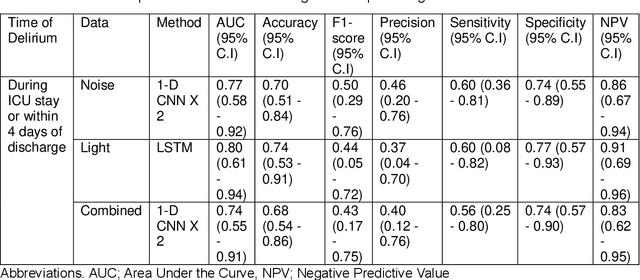
Abstract:Existing Intensive Care Unit (ICU) delirium prediction models do not consider environmental factors despite strong evidence of their influence on delirium. This study reports the first deep-learning based delirium prediction model for ICU patients using only ambient noise and light information. Ambient light and noise intensities were measured from ICU rooms of 102 patients from May 2021 to September 2022 using Thunderboard, ActiGraph sensors and an iPod with AudioTools application. These measurements were divided into daytime (0700 to 1859) and nighttime (1900 to 0659). Deep learning models were trained using this data to predict the incidence of delirium during ICU stay or within 4 days of discharge. Finally, outcome scores were analyzed to evaluate the importance and directionality of every feature. Daytime noise levels were significantly higher than nighttime noise levels. When using only noise features or a combination of noise and light features 1-D convolutional neural networks (CNN) achieved the strongest performance: AUC=0.77, 0.74; Sensitivity=0.60, 0.56; Specificity=0.74, 0.74; Precision=0.46, 0.40 respectively. Using only light features, Long Short-Term Memory (LSTM) networks performed best: AUC=0.80, Sensitivity=0.60, Specificity=0.77, Precision=0.37. Maximum nighttime and minimum daytime noise levels were the strongest positive and negative predictors of delirium respectively. Nighttime light level was a stronger predictor of delirium than daytime light level. Total influence of light features outweighed that of noise features on the second and fourth day of ICU stay. This study shows that ambient light and noise intensities are strong predictors of long-term delirium incidence in the ICU. It reveals that daytime and nighttime environmental factors might influence delirium differently and that the importance of light and noise levels vary over the course of an ICU stay.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge