Tyler Loftus
MANDARIN: Mixture-of-Experts Framework for Dynamic Delirium and Coma Prediction in ICU Patients: Development and Validation of an Acute Brain Dysfunction Prediction Model
Mar 08, 2025Abstract:Acute brain dysfunction (ABD) is a common, severe ICU complication, presenting as delirium or coma and leading to prolonged stays, increased mortality, and cognitive decline. Traditional screening tools like the Glasgow Coma Scale (GCS), Confusion Assessment Method (CAM), and Richmond Agitation-Sedation Scale (RASS) rely on intermittent assessments, causing delays and inconsistencies. In this study, we propose MANDARIN (Mixture-of-Experts Framework for Dynamic Delirium and Coma Prediction in ICU Patients), a 1.5M-parameter mixture-of-experts neural network to predict ABD in real-time among ICU patients. The model integrates temporal and static data from the ICU to predict the brain status in the next 12 to 72 hours, using a multi-branch approach to account for current brain status. The MANDARIN model was trained on data from 92,734 patients (132,997 ICU admissions) from 2 hospitals between 2008-2019 and validated externally on data from 11,719 patients (14,519 ICU admissions) from 15 hospitals and prospectively on data from 304 patients (503 ICU admissions) from one hospital in 2021-2024. Three datasets were used: the University of Florida Health (UFH) dataset, the electronic ICU Collaborative Research Database (eICU), and the Medical Information Mart for Intensive Care (MIMIC)-IV dataset. MANDARIN significantly outperforms the baseline neurological assessment scores (GCS, CAM, and RASS) for delirium prediction in both external (AUROC 75.5% CI: 74.2%-76.8% vs 68.3% CI: 66.9%-69.5%) and prospective (AUROC 82.0% CI: 74.8%-89.2% vs 72.7% CI: 65.5%-81.0%) cohorts, as well as for coma prediction (external AUROC 87.3% CI: 85.9%-89.0% vs 72.8% CI: 70.6%-74.9%, and prospective AUROC 93.4% CI: 88.5%-97.9% vs 67.7% CI: 57.7%-76.8%) with a 12-hour lead time. This tool has the potential to assist clinicians in decision-making by continuously monitoring the brain status of patients in the ICU.
The Potential of Wearable Sensors for Assessing Patient Acuity in Intensive Care Unit (ICU)
Nov 03, 2023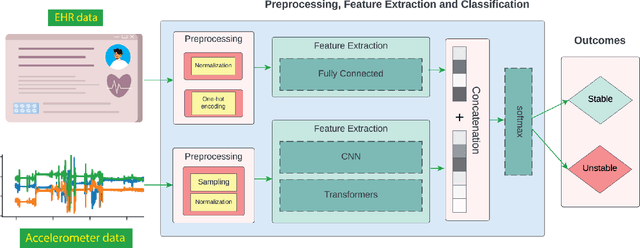
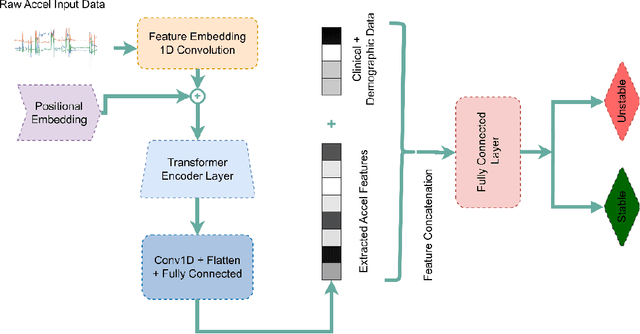

Abstract:Acuity assessments are vital in critical care settings to provide timely interventions and fair resource allocation. Traditional acuity scores rely on manual assessments and documentation of physiological states, which can be time-consuming, intermittent, and difficult to use for healthcare providers. Furthermore, such scores do not incorporate granular information such as patients' mobility level, which can indicate recovery or deterioration in the ICU. We hypothesized that existing acuity scores could be potentially improved by employing Artificial Intelligence (AI) techniques in conjunction with Electronic Health Records (EHR) and wearable sensor data. In this study, we evaluated the impact of integrating mobility data collected from wrist-worn accelerometers with clinical data obtained from EHR for developing an AI-driven acuity assessment score. Accelerometry data were collected from 86 patients wearing accelerometers on their wrists in an academic hospital setting. The data was analyzed using five deep neural network models: VGG, ResNet, MobileNet, SqueezeNet, and a custom Transformer network. These models outperformed a rule-based clinical score (SOFA= Sequential Organ Failure Assessment) used as a baseline, particularly regarding the precision, sensitivity, and F1 score. The results showed that while a model relying solely on accelerometer data achieved limited performance (AUC 0.50, Precision 0.61, and F1-score 0.68), including demographic information with the accelerometer data led to a notable enhancement in performance (AUC 0.69, Precision 0.75, and F1-score 0.67). This work shows that the combination of mobility and patient information can successfully differentiate between stable and unstable states in critically ill patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge