Parisa Rashidi
Intelligent Critical Care Center, University of Florida, Gainesville, FL, Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL
Bridge2AI: Building A Cross-disciplinary Curriculum Towards AI-Enhanced Biomedical and Clinical Care
May 20, 2025Abstract:Objective: As AI becomes increasingly central to healthcare, there is a pressing need for bioinformatics and biomedical training systems that are personalized and adaptable. Materials and Methods: The NIH Bridge2AI Training, Recruitment, and Mentoring (TRM) Working Group developed a cross-disciplinary curriculum grounded in collaborative innovation, ethical data stewardship, and professional development within an adapted Learning Health System (LHS) framework. Results: The curriculum integrates foundational AI modules, real-world projects, and a structured mentee-mentor network spanning Bridge2AI Grand Challenges and the Bridge Center. Guided by six learner personas, the program tailors educational pathways to individual needs while supporting scalability. Discussion: Iterative refinement driven by continuous feedback ensures that content remains responsive to learner progress and emerging trends. Conclusion: With over 30 scholars and 100 mentors engaged across North America, the TRM model demonstrates how adaptive, persona-informed training can build interdisciplinary competencies and foster an integrative, ethically grounded AI education in biomedical contexts.
Quantifying Circadian Desynchrony in ICU Patients and Its Association with Delirium
Mar 11, 2025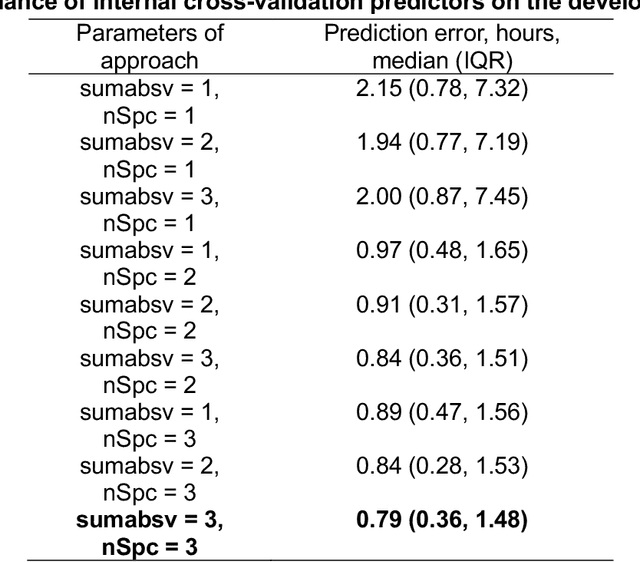
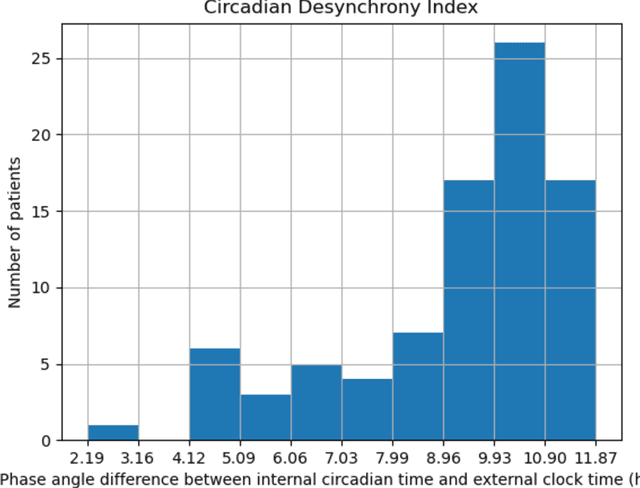


Abstract:Background: Circadian desynchrony characterized by the misalignment between an individual's internal biological rhythms and external environmental cues, significantly affects various physiological processes and health outcomes. Quantifying circadian desynchrony often requires prolonged and frequent monitoring, and currently, an easy tool for this purpose is missing. Additionally, its association with the incidence of delirium has not been clearly explored. Methods: A prospective observational study was carried out in intensive care units (ICU) of a tertiary hospital. Circadian transcriptomics of blood monocytes from 86 individuals were collected on two consecutive days, although a second sample could not be obtained from all participants. Using two public datasets comprised of healthy volunteers, we replicated a model for determining internal circadian time. We developed an approach to quantify circadian desynchrony by comparing internal circadian time and external blood collection time. We applied the model and quantified circadian desynchrony index among ICU patients, and investigated its association with the incidence of delirium. Results: The replicated model for determining internal circadian time achieved comparable high accuracy. The quantified circadian desynchrony index was significantly higher among critically ill ICU patients compared to healthy subjects, with values of 10.03 hours vs 2.50-2.95 hours (p < 0.001). Most ICU patients had a circadian desynchrony index greater than 9 hours. Additionally, the index was lower in patients whose blood samples were drawn after 3pm, with values of 5.00 hours compared to 10.01-10.90 hours in other groups (p < 0.001)...
MANDARIN: Mixture-of-Experts Framework for Dynamic Delirium and Coma Prediction in ICU Patients: Development and Validation of an Acute Brain Dysfunction Prediction Model
Mar 08, 2025Abstract:Acute brain dysfunction (ABD) is a common, severe ICU complication, presenting as delirium or coma and leading to prolonged stays, increased mortality, and cognitive decline. Traditional screening tools like the Glasgow Coma Scale (GCS), Confusion Assessment Method (CAM), and Richmond Agitation-Sedation Scale (RASS) rely on intermittent assessments, causing delays and inconsistencies. In this study, we propose MANDARIN (Mixture-of-Experts Framework for Dynamic Delirium and Coma Prediction in ICU Patients), a 1.5M-parameter mixture-of-experts neural network to predict ABD in real-time among ICU patients. The model integrates temporal and static data from the ICU to predict the brain status in the next 12 to 72 hours, using a multi-branch approach to account for current brain status. The MANDARIN model was trained on data from 92,734 patients (132,997 ICU admissions) from 2 hospitals between 2008-2019 and validated externally on data from 11,719 patients (14,519 ICU admissions) from 15 hospitals and prospectively on data from 304 patients (503 ICU admissions) from one hospital in 2021-2024. Three datasets were used: the University of Florida Health (UFH) dataset, the electronic ICU Collaborative Research Database (eICU), and the Medical Information Mart for Intensive Care (MIMIC)-IV dataset. MANDARIN significantly outperforms the baseline neurological assessment scores (GCS, CAM, and RASS) for delirium prediction in both external (AUROC 75.5% CI: 74.2%-76.8% vs 68.3% CI: 66.9%-69.5%) and prospective (AUROC 82.0% CI: 74.8%-89.2% vs 72.7% CI: 65.5%-81.0%) cohorts, as well as for coma prediction (external AUROC 87.3% CI: 85.9%-89.0% vs 72.8% CI: 70.6%-74.9%, and prospective AUROC 93.4% CI: 88.5%-97.9% vs 67.7% CI: 57.7%-76.8%) with a 12-hour lead time. This tool has the potential to assist clinicians in decision-making by continuously monitoring the brain status of patients in the ICU.
MANGO: Multimodal Acuity traNsformer for intelliGent ICU Outcomes
Dec 13, 2024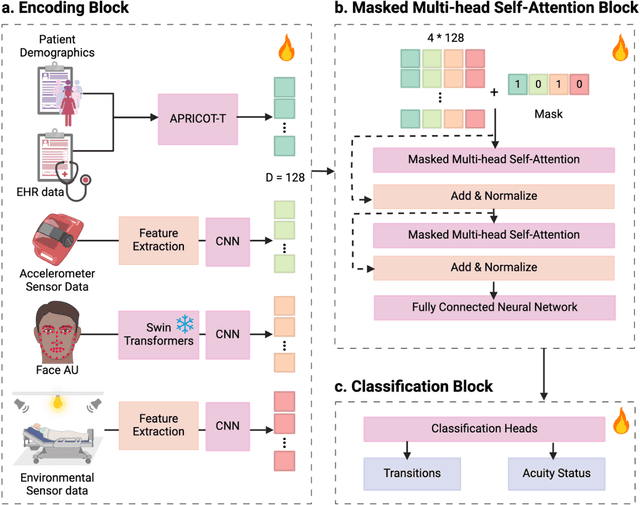
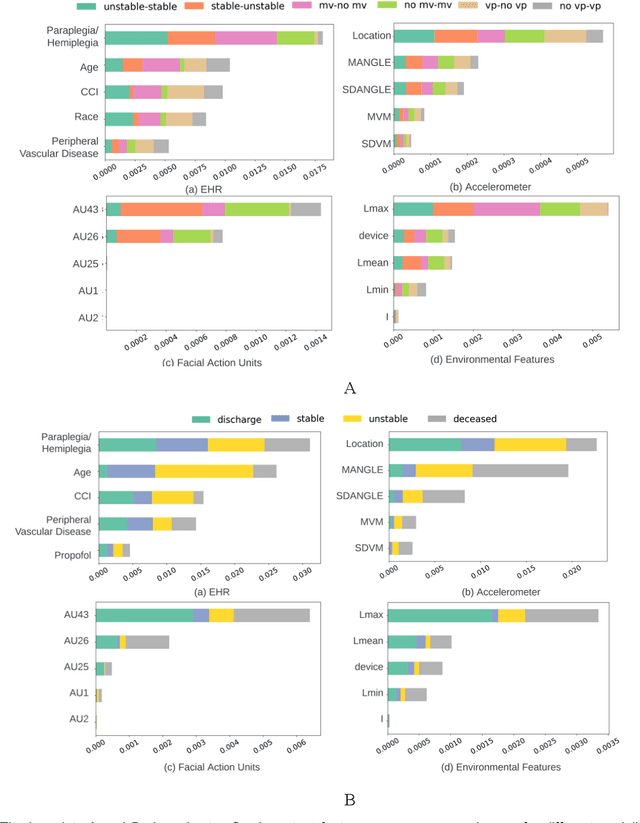
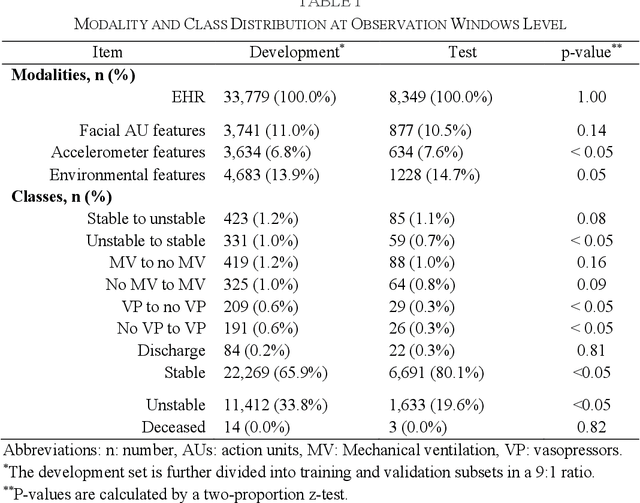
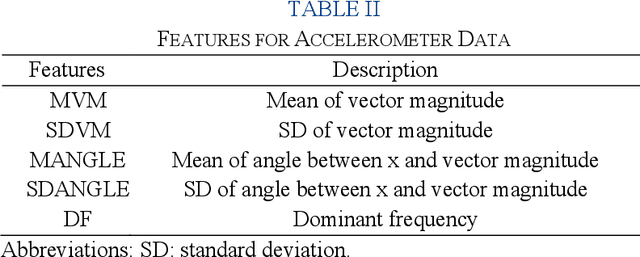
Abstract:Estimation of patient acuity in the Intensive Care Unit (ICU) is vital to ensure timely and appropriate interventions. Advances in artificial intelligence (AI) technologies have significantly improved the accuracy of acuity predictions. However, prior studies using machine learning for acuity prediction have predominantly relied on electronic health records (EHR) data, often overlooking other critical aspects of ICU stay, such as patient mobility, environmental factors, and facial cues indicating pain or agitation. To address this gap, we present MANGO: the Multimodal Acuity traNsformer for intelliGent ICU Outcomes, designed to enhance the prediction of patient acuity states, transitions, and the need for life-sustaining therapy. We collected a multimodal dataset ICU-Multimodal, incorporating four key modalities, EHR data, wearable sensor data, video of patient's facial cues, and ambient sensor data, which we utilized to train MANGO. The MANGO model employs a multimodal feature fusion network powered by Transformer masked self-attention method, enabling it to capture and learn complex interactions across these diverse data modalities even when some modalities are absent. Our results demonstrated that integrating multiple modalities significantly improved the model's ability to predict acuity status, transitions, and the need for life-sustaining therapy. The best-performing models achieved an area under the receiver operating characteristic curve (AUROC) of 0.76 (95% CI: 0.72-0.79) for predicting transitions in acuity status and the need for life-sustaining therapy, while 0.82 (95% CI: 0.69-0.89) for acuity status prediction...
Peri-AIIMS: Perioperative Artificial Intelligence Driven Integrated Modeling of Surgeries using Anesthetic, Physical and Cognitive Statuses for Predicting Hospital Outcomes
Oct 29, 2024



Abstract:The association between preoperative cognitive status and surgical outcomes is a critical, yet scarcely explored area of research. Linking intraoperative data with postoperative outcomes is a promising and low-cost way of evaluating long-term impacts of surgical interventions. In this study, we evaluated how preoperative cognitive status as measured by the clock drawing test contributed to predicting length of hospital stay, hospital charges, average pain experienced during follow-up, and 1-year mortality over and above intraoperative variables, demographics, preoperative physical status and comorbidities. We expanded our analysis to 6 specific surgical groups where sufficient data was available for cross-validation. The clock drawing images were represented by 10 constructional features discovered by a semi-supervised deep learning algorithm, previously validated to differentiate between dementia and non-dementia patients. Different machine learning models were trained to classify postoperative outcomes in hold-out test sets. The models were compared to their relative performance, time complexity, and interpretability. Shapley Additive Explanations (SHAP) analysis was used to find the most predictive features for classifying different outcomes in different surgical contexts. Relative classification performances achieved by different feature sets showed that the perioperative cognitive dataset which included clock drawing features in addition to intraoperative variables, demographics, and comorbidities served as the best dataset for 12 of 18 possible surgery-outcome combinations...
DeLLiriuM: A large language model for delirium prediction in the ICU using structured EHR
Oct 22, 2024



Abstract:Delirium is an acute confusional state that has been shown to affect up to 31% of patients in the intensive care unit (ICU). Early detection of this condition could lead to more timely interventions and improved health outcomes. While artificial intelligence (AI) models have shown great potential for ICU delirium prediction using structured electronic health records (EHR), most of them have not explored the use of state-of-the-art AI models, have been limited to single hospitals, or have been developed and validated on small cohorts. The use of large language models (LLM), models with hundreds of millions to billions of parameters, with structured EHR data could potentially lead to improved predictive performance. In this study, we propose DeLLiriuM, a novel LLM-based delirium prediction model using EHR data available in the first 24 hours of ICU admission to predict the probability of a patient developing delirium during the rest of their ICU admission. We develop and validate DeLLiriuM on ICU admissions from 104,303 patients pertaining to 195 hospitals across three large databases: the eICU Collaborative Research Database, the Medical Information Mart for Intensive Care (MIMIC)-IV, and the University of Florida Health's Integrated Data Repository. The performance measured by the area under the receiver operating characteristic curve (AUROC) showed that DeLLiriuM outperformed all baselines in two external validation sets, with 0.77 (95% confidence interval 0.76-0.78) and 0.84 (95% confidence interval 0.83-0.85) across 77,543 patients spanning 194 hospitals. To the best of our knowledge, DeLLiriuM is the first LLM-based delirium prediction tool for the ICU based on structured EHR data, outperforming deep learning baselines which employ structured features and can provide helpful information to clinicians for timely interventions.
Transparent AI: Developing an Explainable Interface for Predicting Postoperative Complications
Apr 18, 2024



Abstract:Given the sheer volume of surgical procedures and the significant rate of postoperative fatalities, assessing and managing surgical complications has become a critical public health concern. Existing artificial intelligence (AI) tools for risk surveillance and diagnosis often lack adequate interpretability, fairness, and reproducibility. To address this, we proposed an Explainable AI (XAI) framework designed to answer five critical questions: why, why not, how, what if, and what else, with the goal of enhancing the explainability and transparency of AI models. We incorporated various techniques such as Local Interpretable Model-agnostic Explanations (LIME), SHapley Additive exPlanations (SHAP), counterfactual explanations, model cards, an interactive feature manipulation interface, and the identification of similar patients to address these questions. We showcased an XAI interface prototype that adheres to this framework for predicting major postoperative complications. This initial implementation has provided valuable insights into the vast explanatory potential of our XAI framework and represents an initial step towards its clinical adoption.
Global Contrastive Training for Multimodal Electronic Health Records with Language Supervision
Apr 10, 2024
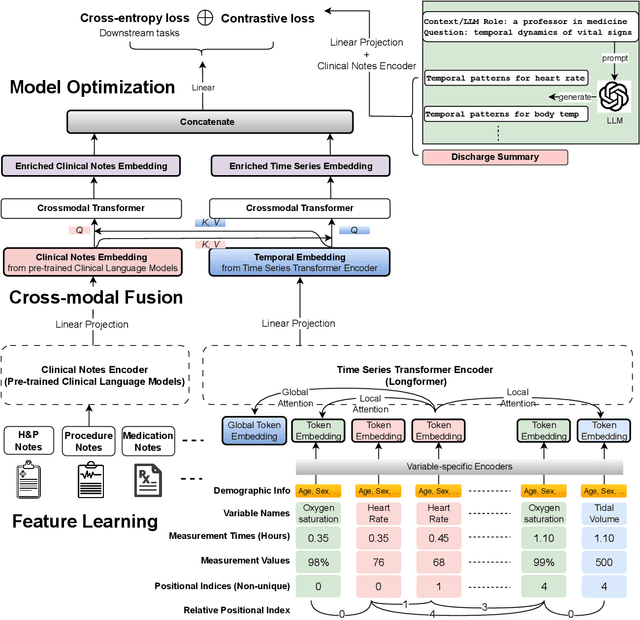
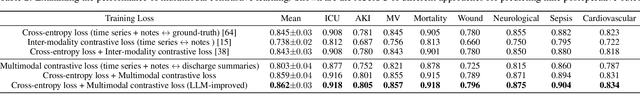
Abstract:Modern electronic health records (EHRs) hold immense promise in tracking personalized patient health trajectories through sequential deep learning, owing to their extensive breadth, scale, and temporal granularity. Nonetheless, how to effectively leverage multiple modalities from EHRs poses significant challenges, given its complex characteristics such as high dimensionality, multimodality, sparsity, varied recording frequencies, and temporal irregularities. To this end, this paper introduces a novel multimodal contrastive learning framework, specifically focusing on medical time series and clinical notes. To tackle the challenge of sparsity and irregular time intervals in medical time series, the framework integrates temporal cross-attention transformers with a dynamic embedding and tokenization scheme for learning multimodal feature representations. To harness the interconnected relationships between medical time series and clinical notes, the framework equips a global contrastive loss, aligning a patient's multimodal feature representations with the corresponding discharge summaries. Since discharge summaries uniquely pertain to individual patients and represent a holistic view of the patient's hospital stay, machine learning models are led to learn discriminative multimodal features via global contrasting. Extensive experiments with a real-world EHR dataset demonstrated that our framework outperformed state-of-the-art approaches on the exemplar task of predicting the occurrence of nine postoperative complications for more than 120,000 major inpatient surgeries using multimodal data from UF health system split among three hospitals (UF Health Gainesville, UF Health Jacksonville, and UF Health Jacksonville-North).
Federated learning model for predicting major postoperative complications
Apr 09, 2024
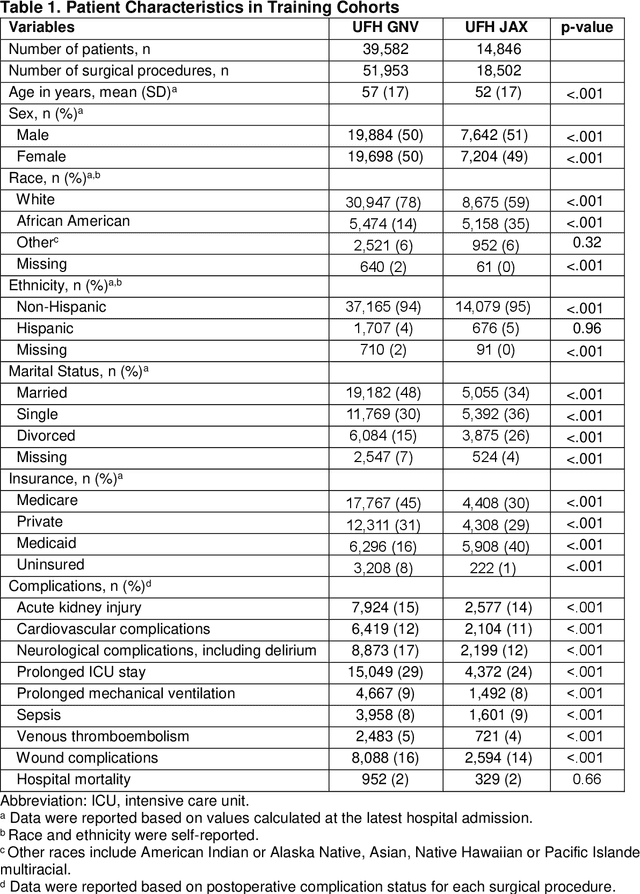


Abstract:Background: The accurate prediction of postoperative complication risk using Electronic Health Records (EHR) and artificial intelligence shows great potential. Training a robust artificial intelligence model typically requires large-scale and diverse datasets. In reality, collecting medical data often encounters challenges surrounding privacy protection. Methods: This retrospective cohort study includes adult patients who were admitted to UFH Gainesville (GNV) (n = 79,850) and Jacksonville (JAX) (n = 28,636) for any type of inpatient surgical procedure. Using perioperative and intraoperative features, we developed federated learning models to predict nine major postoperative complications (i.e., prolonged intensive care unit stay and mechanical ventilation). We compared federated learning models with local learning models trained on a single site and central learning models trained on pooled dataset from two centers. Results: Our federated learning models achieved the area under the receiver operating characteristics curve (AUROC) values ranged from 0.81 for wound complications to 0.92 for prolonged ICU stay at UFH GNV center. At UFH JAX center, these values ranged from 0.73-0.74 for wound complications to 0.92-0.93 for hospital mortality. Federated learning models achieved comparable AUROC performance to central learning models, except for prolonged ICU stay, where the performance of federated learning models was slightly higher than central learning models at UFH GNV center, but slightly lower at UFH JAX center. In addition, our federated learning model obtained comparable performance to the best local learning model at each center, demonstrating strong generalizability. Conclusion: Federated learning is shown to be a useful tool to train robust and generalizable models from large scale data across multiple institutions where data protection barriers are high.
A multi-cohort study on prediction of acute brain dysfunction states using selective state space models
Mar 11, 2024

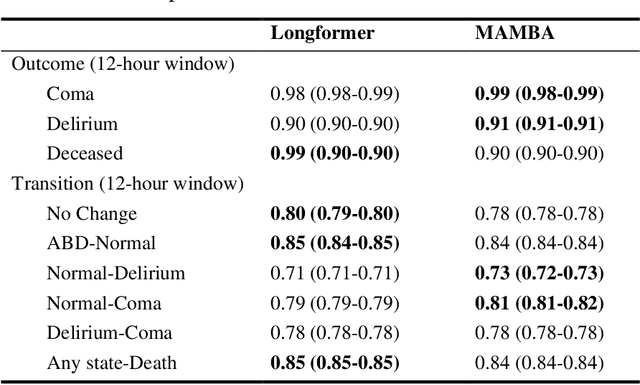

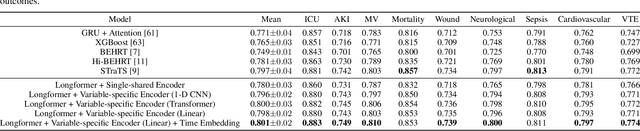
Abstract:Assessing acute brain dysfunction (ABD), including delirium and coma in the intensive care unit (ICU), is a critical challenge due to its prevalence and severe implications for patient outcomes. Current diagnostic methods rely on infrequent clinical observations, which can only determine a patient's ABD status after onset. Our research attempts to solve these problems by harnessing Electronic Health Records (EHR) data to develop automated methods for ABD prediction for patients in the ICU. Existing models solely predict a single state (e.g., either delirium or coma), require at least 24 hours of observation data to make predictions, do not dynamically predict fluctuating ABD conditions during ICU stay (typically a one-time prediction), and use small sample size, proprietary single-hospital datasets. Our research fills these gaps in the existing literature by dynamically predicting delirium, coma, and mortality for 12-hour intervals throughout an ICU stay and validating on two public datasets. Our research also introduces the concept of dynamically predicting critical transitions from non-ABD to ABD and between different ABD states in real time, which could be clinically more informative for the hospital staff. We compared the predictive performance of two state-of-the-art neural network models, the MAMBA selective state space model and the Longformer Transformer model. Using the MAMBA model, we achieved a mean area under the receiving operator characteristic curve (AUROC) of 0.95 on outcome prediction of ABD for 12-hour intervals. The model achieves a mean AUROC of 0.79 when predicting transitions between ABD states. Our study uses a curated dataset from the University of Florida Health Shands Hospital for internal validation and two publicly available datasets, MIMIC-IV and eICU, for external validation, demonstrating robustness across ICU stays from 203 hospitals and 140,945 patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge