Tyler J. Loftus
Intelligent Critical Care Center, University of Florida, Gainesville, FL, Department of Surgery, College of Medicine, University of Florida, Gainesville, FL
MANGO: Multimodal Acuity traNsformer for intelliGent ICU Outcomes
Dec 13, 2024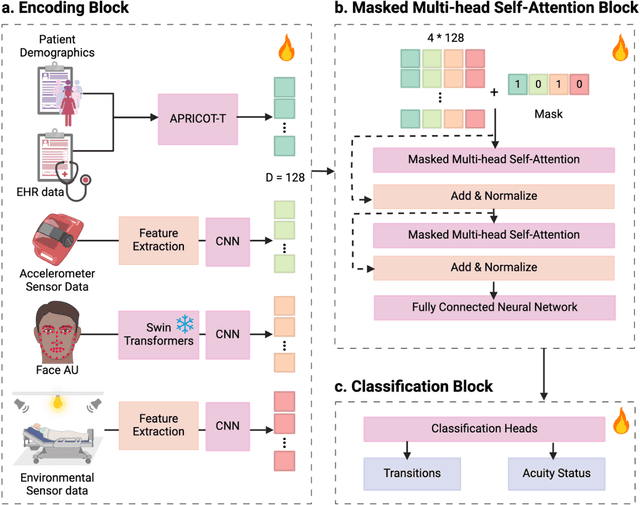
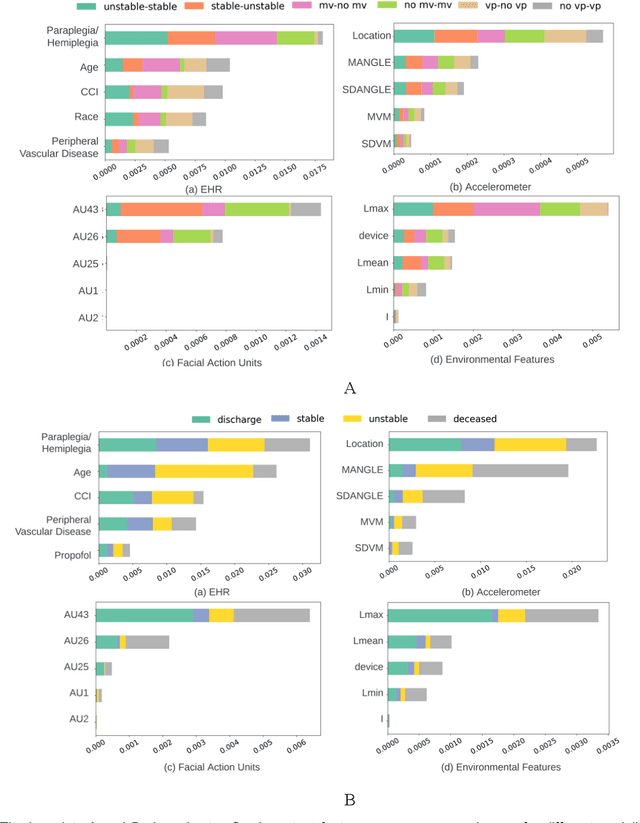
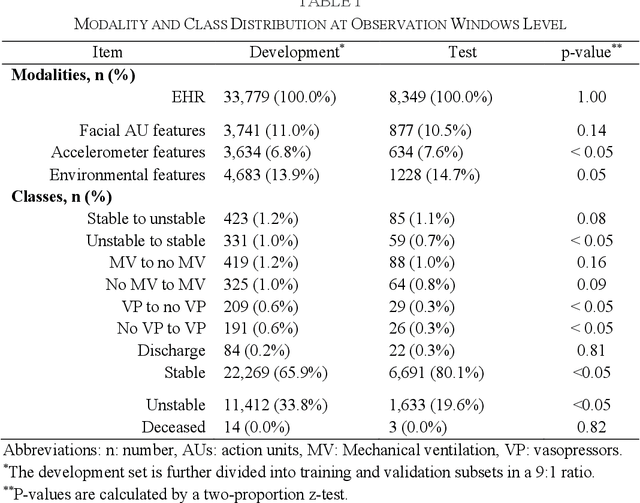
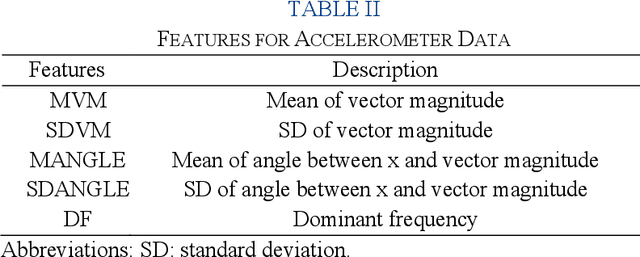
Abstract:Estimation of patient acuity in the Intensive Care Unit (ICU) is vital to ensure timely and appropriate interventions. Advances in artificial intelligence (AI) technologies have significantly improved the accuracy of acuity predictions. However, prior studies using machine learning for acuity prediction have predominantly relied on electronic health records (EHR) data, often overlooking other critical aspects of ICU stay, such as patient mobility, environmental factors, and facial cues indicating pain or agitation. To address this gap, we present MANGO: the Multimodal Acuity traNsformer for intelliGent ICU Outcomes, designed to enhance the prediction of patient acuity states, transitions, and the need for life-sustaining therapy. We collected a multimodal dataset ICU-Multimodal, incorporating four key modalities, EHR data, wearable sensor data, video of patient's facial cues, and ambient sensor data, which we utilized to train MANGO. The MANGO model employs a multimodal feature fusion network powered by Transformer masked self-attention method, enabling it to capture and learn complex interactions across these diverse data modalities even when some modalities are absent. Our results demonstrated that integrating multiple modalities significantly improved the model's ability to predict acuity status, transitions, and the need for life-sustaining therapy. The best-performing models achieved an area under the receiver operating characteristic curve (AUROC) of 0.76 (95% CI: 0.72-0.79) for predicting transitions in acuity status and the need for life-sustaining therapy, while 0.82 (95% CI: 0.69-0.89) for acuity status prediction...
Transparent AI: Developing an Explainable Interface for Predicting Postoperative Complications
Apr 18, 2024



Abstract:Given the sheer volume of surgical procedures and the significant rate of postoperative fatalities, assessing and managing surgical complications has become a critical public health concern. Existing artificial intelligence (AI) tools for risk surveillance and diagnosis often lack adequate interpretability, fairness, and reproducibility. To address this, we proposed an Explainable AI (XAI) framework designed to answer five critical questions: why, why not, how, what if, and what else, with the goal of enhancing the explainability and transparency of AI models. We incorporated various techniques such as Local Interpretable Model-agnostic Explanations (LIME), SHapley Additive exPlanations (SHAP), counterfactual explanations, model cards, an interactive feature manipulation interface, and the identification of similar patients to address these questions. We showcased an XAI interface prototype that adheres to this framework for predicting major postoperative complications. This initial implementation has provided valuable insights into the vast explanatory potential of our XAI framework and represents an initial step towards its clinical adoption.
Global Contrastive Training for Multimodal Electronic Health Records with Language Supervision
Apr 10, 2024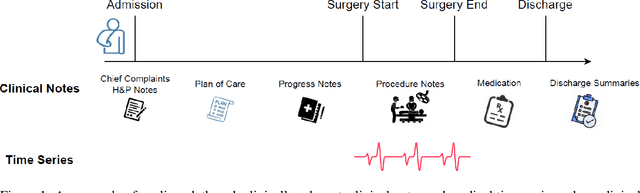
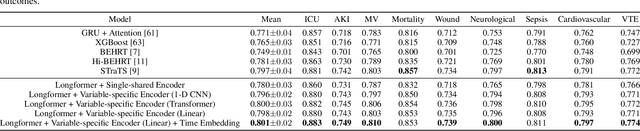
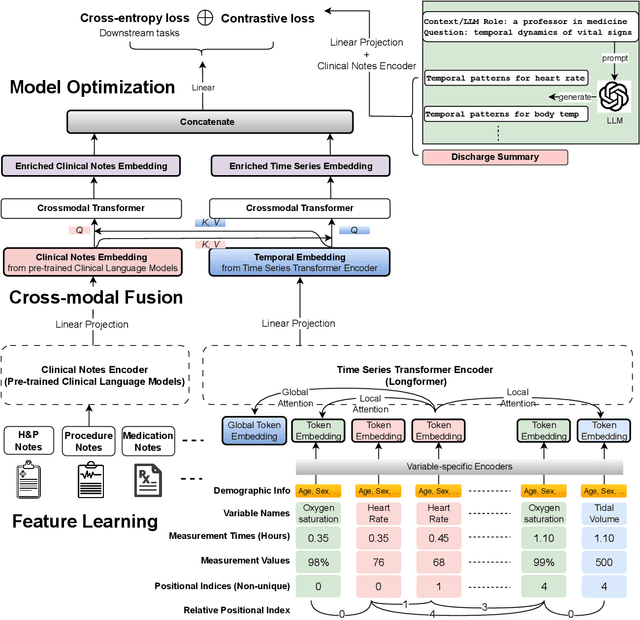
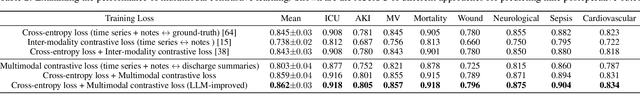
Abstract:Modern electronic health records (EHRs) hold immense promise in tracking personalized patient health trajectories through sequential deep learning, owing to their extensive breadth, scale, and temporal granularity. Nonetheless, how to effectively leverage multiple modalities from EHRs poses significant challenges, given its complex characteristics such as high dimensionality, multimodality, sparsity, varied recording frequencies, and temporal irregularities. To this end, this paper introduces a novel multimodal contrastive learning framework, specifically focusing on medical time series and clinical notes. To tackle the challenge of sparsity and irregular time intervals in medical time series, the framework integrates temporal cross-attention transformers with a dynamic embedding and tokenization scheme for learning multimodal feature representations. To harness the interconnected relationships between medical time series and clinical notes, the framework equips a global contrastive loss, aligning a patient's multimodal feature representations with the corresponding discharge summaries. Since discharge summaries uniquely pertain to individual patients and represent a holistic view of the patient's hospital stay, machine learning models are led to learn discriminative multimodal features via global contrasting. Extensive experiments with a real-world EHR dataset demonstrated that our framework outperformed state-of-the-art approaches on the exemplar task of predicting the occurrence of nine postoperative complications for more than 120,000 major inpatient surgeries using multimodal data from UF health system split among three hospitals (UF Health Gainesville, UF Health Jacksonville, and UF Health Jacksonville-North).
Federated learning model for predicting major postoperative complications
Apr 09, 2024


Abstract:Background: The accurate prediction of postoperative complication risk using Electronic Health Records (EHR) and artificial intelligence shows great potential. Training a robust artificial intelligence model typically requires large-scale and diverse datasets. In reality, collecting medical data often encounters challenges surrounding privacy protection. Methods: This retrospective cohort study includes adult patients who were admitted to UFH Gainesville (GNV) (n = 79,850) and Jacksonville (JAX) (n = 28,636) for any type of inpatient surgical procedure. Using perioperative and intraoperative features, we developed federated learning models to predict nine major postoperative complications (i.e., prolonged intensive care unit stay and mechanical ventilation). We compared federated learning models with local learning models trained on a single site and central learning models trained on pooled dataset from two centers. Results: Our federated learning models achieved the area under the receiver operating characteristics curve (AUROC) values ranged from 0.81 for wound complications to 0.92 for prolonged ICU stay at UFH GNV center. At UFH JAX center, these values ranged from 0.73-0.74 for wound complications to 0.92-0.93 for hospital mortality. Federated learning models achieved comparable AUROC performance to central learning models, except for prolonged ICU stay, where the performance of federated learning models was slightly higher than central learning models at UFH GNV center, but slightly lower at UFH JAX center. In addition, our federated learning model obtained comparable performance to the best local learning model at each center, demonstrating strong generalizability. Conclusion: Federated learning is shown to be a useful tool to train robust and generalizable models from large scale data across multiple institutions where data protection barriers are high.
Temporal Cross-Attention for Dynamic Embedding and Tokenization of Multimodal Electronic Health Records
Mar 06, 2024Abstract:The breadth, scale, and temporal granularity of modern electronic health records (EHR) systems offers great potential for estimating personalized and contextual patient health trajectories using sequential deep learning. However, learning useful representations of EHR data is challenging due to its high dimensionality, sparsity, multimodality, irregular and variable-specific recording frequency, and timestamp duplication when multiple measurements are recorded simultaneously. Although recent efforts to fuse structured EHR and unstructured clinical notes suggest the potential for more accurate prediction of clinical outcomes, less focus has been placed on EHR embedding approaches that directly address temporal EHR challenges by learning time-aware representations from multimodal patient time series. In this paper, we introduce a dynamic embedding and tokenization framework for precise representation of multimodal clinical time series that combines novel methods for encoding time and sequential position with temporal cross-attention. Our embedding and tokenization framework, when integrated into a multitask transformer classifier with sliding window attention, outperformed baseline approaches on the exemplar task of predicting the occurrence of nine postoperative complications of more than 120,000 major inpatient surgeries using multimodal data from three hospitals and two academic health centers in the United States.
Identifying acute illness phenotypes via deep temporal interpolation and clustering network on physiologic signatures
Jul 27, 2023Abstract:Initial hours of hospital admission impact clinical trajectory, but early clinical decisions often suffer due to data paucity. With clustering analysis for vital signs within six hours of admission, patient phenotypes with distinct pathophysiological signatures and outcomes may support early clinical decisions. We created a single-center, longitudinal EHR dataset for 75,762 adults admitted to a tertiary care center for 6+ hours. We proposed a deep temporal interpolation and clustering network to extract latent representations from sparse, irregularly sampled vital sign data and derived distinct patient phenotypes in a training cohort (n=41,502). Model and hyper-parameters were chosen based on a validation cohort (n=17,415). Test cohort (n=16,845) was used to analyze reproducibility and correlation with biomarkers. The training, validation, and testing cohorts had similar distributions of age (54-55 yrs), sex (55% female), race, comorbidities, and illness severity. Four clusters were identified. Phenotype A (18%) had most comorbid disease with higher rate of prolonged respiratory insufficiency, acute kidney injury, sepsis, and three-year mortality. Phenotypes B (33%) and C (31%) had diffuse patterns of mild organ dysfunction. Phenotype B had favorable short-term outcomes but second-highest three-year mortality. Phenotype C had favorable clinical outcomes. Phenotype D (17%) had early/persistent hypotension, high rate of early surgery, and substantial biomarker rate of inflammation but second-lowest three-year mortality. After comparing phenotypes' SOFA scores, clustering results did not simply repeat other acuity assessments. In a heterogeneous cohort, four phenotypes with distinct categories of disease and outcomes were identified by a deep temporal interpolation and clustering network. This tool may impact triage decisions and clinical decision-support under time constraints.
Computable Phenotypes to Characterize Changing Patient Brain Dysfunction in the Intensive Care Unit
Mar 09, 2023



Abstract:In the United States, more than 5 million patients are admitted annually to ICUs, with ICU mortality of 10%-29% and costs over $82 billion. Acute brain dysfunction status, delirium, is often underdiagnosed or undervalued. This study's objective was to develop automated computable phenotypes for acute brain dysfunction states and describe transitions among brain dysfunction states to illustrate the clinical trajectories of ICU patients. We created two single-center, longitudinal EHR datasets for 48,817 adult patients admitted to an ICU at UFH Gainesville (GNV) and Jacksonville (JAX). We developed algorithms to quantify acute brain dysfunction status including coma, delirium, normal, or death at 12-hour intervals of each ICU admission and to identify acute brain dysfunction phenotypes using continuous acute brain dysfunction status and k-means clustering approach. There were 49,770 admissions for 37,835 patients in UFH GNV dataset and 18,472 admissions for 10,982 patients in UFH JAX dataset. In total, 18% of patients had coma as the worst brain dysfunction status; every 12 hours, around 4%-7% would transit to delirium, 22%-25% would recover, 3%-4% would expire, and 67%-68% would remain in a coma in the ICU. Additionally, 7% of patients had delirium as the worst brain dysfunction status; around 6%-7% would transit to coma, 40%-42% would be no delirium, 1% would expire, and 51%-52% would remain delirium in the ICU. There were three phenotypes: persistent coma/delirium, persistently normal, and transition from coma/delirium to normal almost exclusively in first 48 hours after ICU admission. We developed phenotyping scoring algorithms that determined acute brain dysfunction status every 12 hours while admitted to the ICU. This approach may be useful in developing prognostic and decision-support tools to aid patients and clinicians in decision-making on resource use and escalation of care.
Application of Deep Interpolation Network for Clustering of Physiologic Time Series
Apr 27, 2020



Abstract:Background: During the early stages of hospital admission, clinicians must use limited information to make diagnostic and treatment decisions as patient acuity evolves. However, it is common that the time series vital sign information from patients to be both sparse and irregularly collected, which poses a significant challenge for machine / deep learning techniques to analyze and facilitate the clinicians to improve the human health outcome. To deal with this problem, We propose a novel deep interpolation network to extract latent representations from sparse and irregularly sampled time-series vital signs measured within six hours of hospital admission. Methods: We created a single-center longitudinal dataset of electronic health record data for all (n=75,762) adult patient admissions to a tertiary care center lasting six hours or longer, using 55% of the dataset for training, 23% for validation, and 22% for testing. All raw time series within six hours of hospital admission were extracted for six vital signs (systolic blood pressure, diastolic blood pressure, heart rate, temperature, blood oxygen saturation, and respiratory rate). A deep interpolation network is proposed to learn from such irregular and sparse multivariate time series data to extract the fixed low-dimensional latent patterns. We use k-means clustering algorithm to clusters the patient admissions resulting into 7 clusters. Findings: Training, validation, and testing cohorts had similar age (55-57 years), sex (55% female), and admission vital signs. Seven distinct clusters were identified. M Interpretation: In a heterogeneous cohort of hospitalized patients, a deep interpolation network extracted representations from vital sign data measured within six hours of hospital admission. This approach may have important implications for clinical decision-support under time constraints and uncertainty.
Development of Computable Phenotype to Identify and Characterize Transitions in Acuity Status in Intensive Care Unit
Apr 27, 2020



Abstract:Background: In the United States, 5.7 million patients are admitted annually to intensive care units (ICU), with costs exceeding $82 billion. Although close monitoring and dynamic assessment of patient acuity are key aspects of ICU care, both are limited by the time constraints imposed on healthcare providers. Methods: Using the University of Florida Health (UFH) Integrated Data Repository as Honest Broker, we created a database with electronic health records data from a retrospective study cohort of 38,749 adult patients admitted to ICU at UF Health between 06/01/2014 and 08/22/2019. This repository includes demographic information, comorbidities, vital signs, laboratory values, medications with date and timestamps, and diagnoses and procedure codes for all index admission encounters as well as encounters within 12 months prior to index admission and 12 months follow-up. We developed algorithms to identify acuity status of the patient every four hours during each ICU stay. Results: We had 383,193 encounters (121,800 unique patients) admitted to the hospital, and 51,073 encounters (38,749 unique patients) with at least one ICU stay that lasted more than four hours. These patients requiring ICU admission had longer median hospital stay (7 days vs. 1 day) and higher in-hospital mortality (9.6% vs. 0.4%) compared with those not admitted to the ICU. Among patients who were admitted to the ICU and expired during hospital admission, more deaths occurred in the ICU than on general hospital wards (7.4% vs. 0.8%, respectively). Conclusions: We developed phenotyping algorithms that determined patient acuity status every four hours while admitted to the ICU. This approach may be useful in developing prognostic and clinical decision-support tools to aid patients, caregivers, and providers in shared decision-making processes regarding resource use and escalation of care.
Interpretable Multi-Task Deep Neural Networks for Dynamic Predictions of Postoperative Complications
Apr 27, 2020



Abstract:Accurate prediction of postoperative complications can inform shared decisions between patients and surgeons regarding the appropriateness of surgery, preoperative risk-reduction strategies, and postoperative resource use. Traditional predictive analytic tools are hindered by suboptimal performance and usability. We hypothesized that novel deep learning techniques would outperform logistic regression models in predicting postoperative complications. In a single-center longitudinal cohort of 43,943 adult patients undergoing 52,529 major inpatient surgeries, deep learning yielded greater discrimination than logistic regression for all nine complications. Predictive performance was strongest when leveraging the full spectrum of preoperative and intraoperative physiologic time-series electronic health record data. A single multi-task deep learning model yielded greater performance than separate models trained on individual complications. Integrated gradients interpretability mechanisms demonstrated the substantial importance of missing data. Interpretable, multi-task deep neural networks made accurate, patient-level predictions that harbor the potential to augment surgical decision-making.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge