Jonathan Krause
Capabilities of Gemini Models in Medicine
May 01, 2024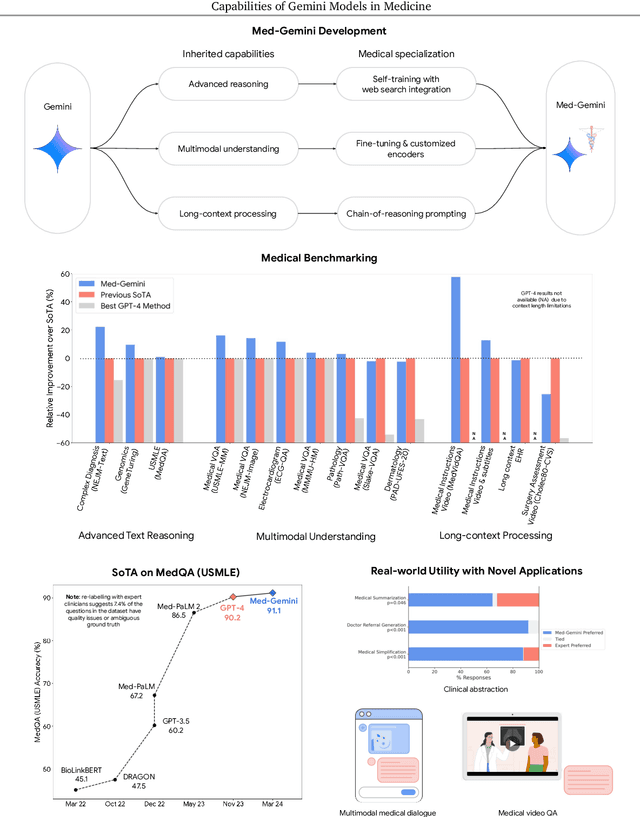
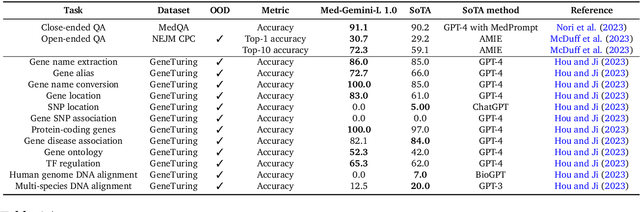
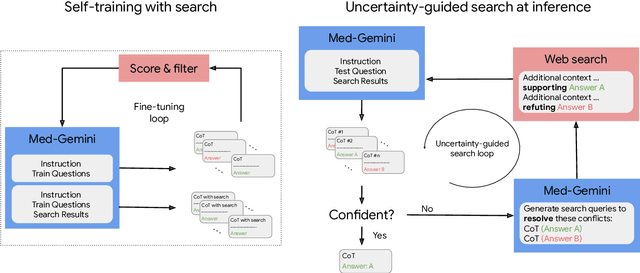
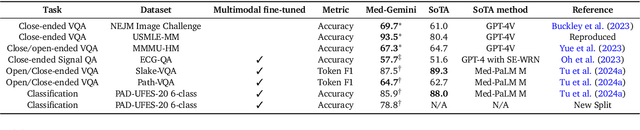
Abstract:Excellence in a wide variety of medical applications poses considerable challenges for AI, requiring advanced reasoning, access to up-to-date medical knowledge and understanding of complex multimodal data. Gemini models, with strong general capabilities in multimodal and long-context reasoning, offer exciting possibilities in medicine. Building on these core strengths of Gemini, we introduce Med-Gemini, a family of highly capable multimodal models that are specialized in medicine with the ability to seamlessly use web search, and that can be efficiently tailored to novel modalities using custom encoders. We evaluate Med-Gemini on 14 medical benchmarks, establishing new state-of-the-art (SoTA) performance on 10 of them, and surpass the GPT-4 model family on every benchmark where a direct comparison is viable, often by a wide margin. On the popular MedQA (USMLE) benchmark, our best-performing Med-Gemini model achieves SoTA performance of 91.1% accuracy, using a novel uncertainty-guided search strategy. On 7 multimodal benchmarks including NEJM Image Challenges and MMMU (health & medicine), Med-Gemini improves over GPT-4V by an average relative margin of 44.5%. We demonstrate the effectiveness of Med-Gemini's long-context capabilities through SoTA performance on a needle-in-a-haystack retrieval task from long de-identified health records and medical video question answering, surpassing prior bespoke methods using only in-context learning. Finally, Med-Gemini's performance suggests real-world utility by surpassing human experts on tasks such as medical text summarization, alongside demonstrations of promising potential for multimodal medical dialogue, medical research and education. Taken together, our results offer compelling evidence for Med-Gemini's potential, although further rigorous evaluation will be crucial before real-world deployment in this safety-critical domain.
Domain-specific optimization and diverse evaluation of self-supervised models for histopathology
Oct 20, 2023
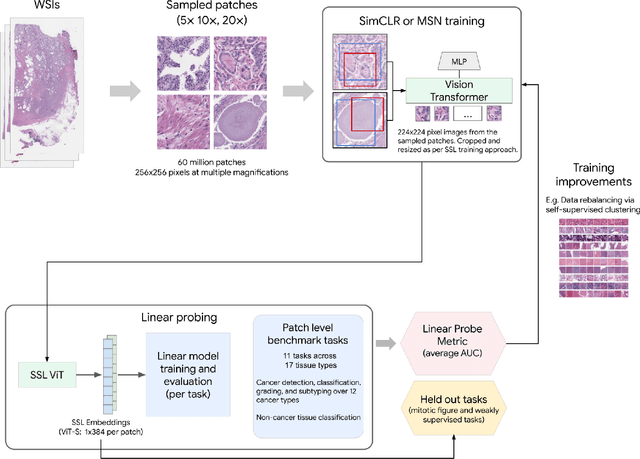
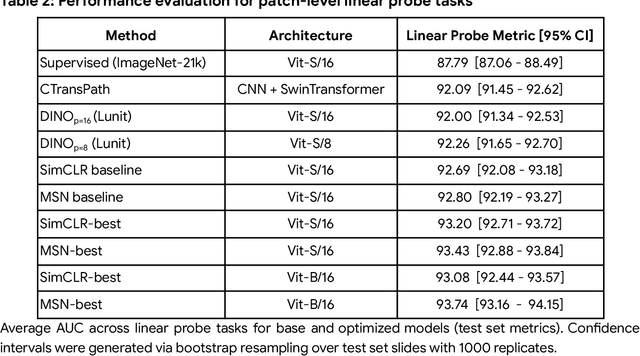
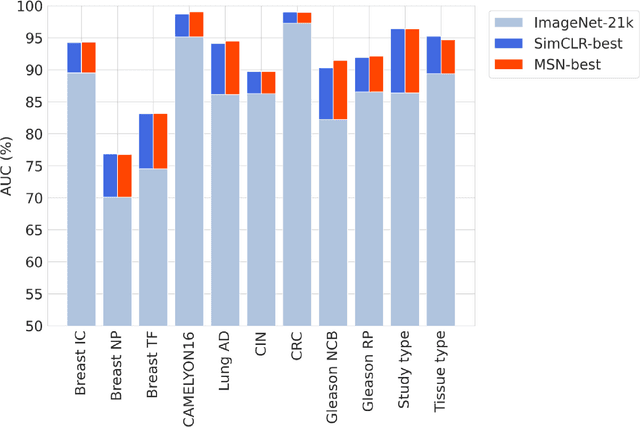
Abstract:Task-specific deep learning models in histopathology offer promising opportunities for improving diagnosis, clinical research, and precision medicine. However, development of such models is often limited by availability of high-quality data. Foundation models in histopathology that learn general representations across a wide range of tissue types, diagnoses, and magnifications offer the potential to reduce the data, compute, and technical expertise necessary to develop task-specific deep learning models with the required level of model performance. In this work, we describe the development and evaluation of foundation models for histopathology via self-supervised learning (SSL). We first establish a diverse set of benchmark tasks involving 17 unique tissue types and 12 unique cancer types and spanning different optimal magnifications and task types. Next, we use this benchmark to explore and evaluate histopathology-specific SSL methods followed by further evaluation on held out patch-level and weakly supervised tasks. We found that standard SSL methods thoughtfully applied to histopathology images are performant across our benchmark tasks and that domain-specific methodological improvements can further increase performance. Our findings reinforce the value of using domain-specific SSL methods in pathology, and establish a set of high quality foundation models to enable further research across diverse applications.
Discovering novel systemic biomarkers in photos of the external eye
Jul 19, 2022
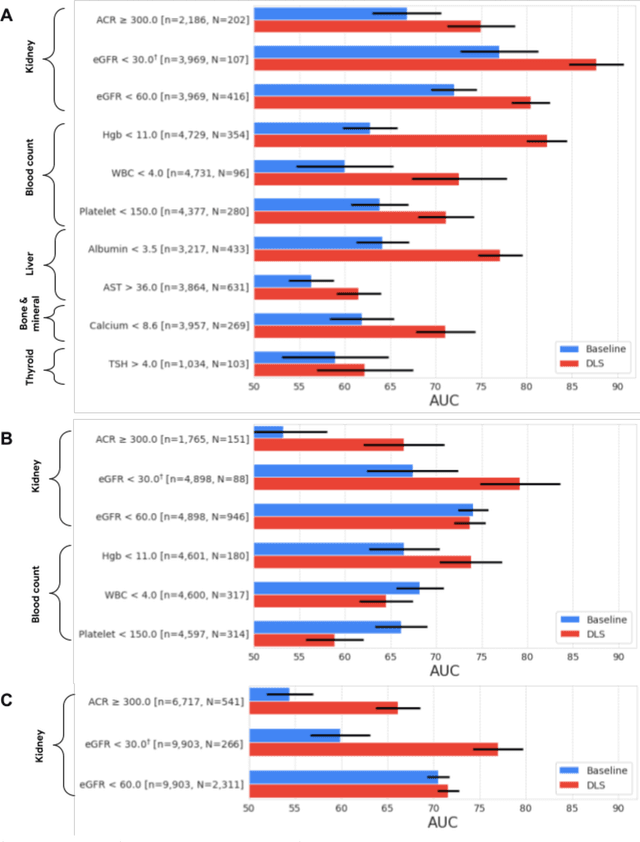
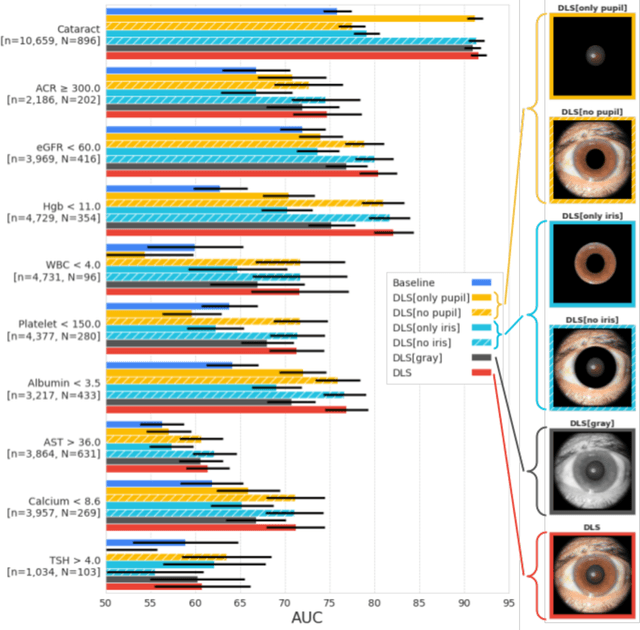
Abstract:External eye photos were recently shown to reveal signs of diabetic retinal disease and elevated HbA1c. In this paper, we evaluate if external eye photos contain information about additional systemic medical conditions. We developed a deep learning system (DLS) that takes external eye photos as input and predicts multiple systemic parameters, such as those related to the liver (albumin, AST); kidney (eGFR estimated using the race-free 2021 CKD-EPI creatinine equation, the urine ACR); bone & mineral (calcium); thyroid (TSH); and blood count (Hgb, WBC, platelets). Development leveraged 151,237 images from 49,015 patients with diabetes undergoing diabetic eye screening in 11 sites across Los Angeles county, CA. Evaluation focused on 9 pre-specified systemic parameters and leveraged 3 validation sets (A, B, C) spanning 28,869 patients with and without diabetes undergoing eye screening in 3 independent sites in Los Angeles County, CA, and the greater Atlanta area, GA. We compared against baseline models incorporating available clinicodemographic variables (e.g. age, sex, race/ethnicity, years with diabetes). Relative to the baseline, the DLS achieved statistically significant superior performance at detecting AST>36, calcium<8.6, eGFR<60, Hgb<11, platelets<150, ACR>=300, and WBC<4 on validation set A (a patient population similar to the development sets), where the AUC of DLS exceeded that of the baseline by 5.2-19.4%. On validation sets B and C, with substantial patient population differences compared to the development sets, the DLS outperformed the baseline for ACR>=300 and Hgb<11 by 7.3-13.2%. Our findings provide further evidence that external eye photos contain important biomarkers of systemic health spanning multiple organ systems. Further work is needed to investigate whether and how these biomarkers can be translated into clinical impact.
Improving Medical Annotation Quality to Decrease Labeling Burden Using Stratified Noisy Cross-Validation
Sep 22, 2020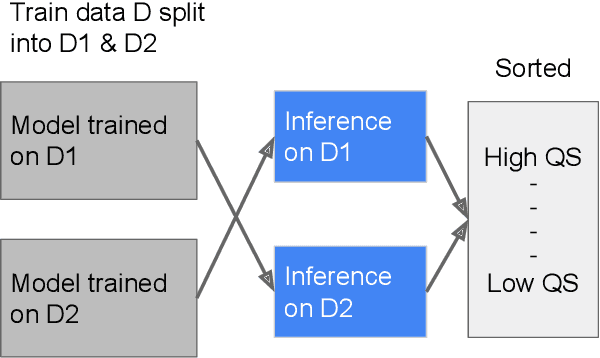

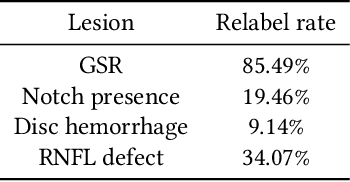
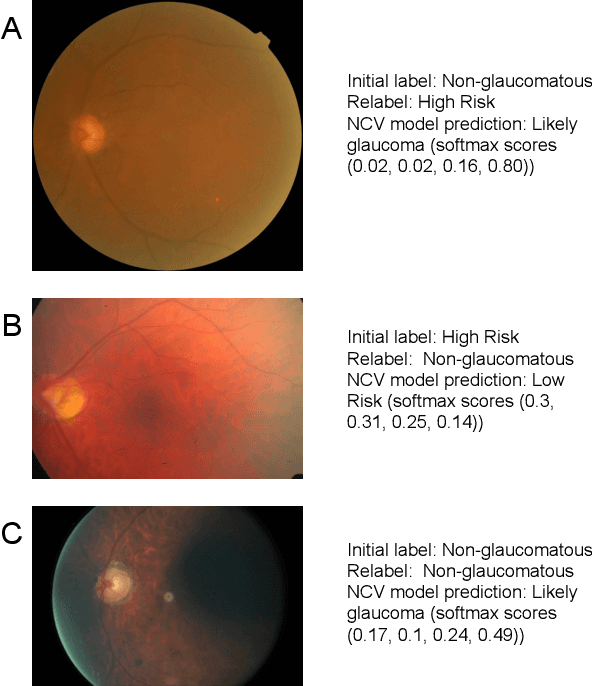
Abstract:As machine learning has become increasingly applied to medical imaging data, noise in training labels has emerged as an important challenge. Variability in diagnosis of medical images is well established; in addition, variability in training and attention to task among medical labelers may exacerbate this issue. Methods for identifying and mitigating the impact of low quality labels have been studied, but are not well characterized in medical imaging tasks. For instance, Noisy Cross-Validation splits the training data into halves, and has been shown to identify low-quality labels in computer vision tasks; but it has not been applied to medical imaging tasks specifically. In this work we introduce Stratified Noisy Cross-Validation (SNCV), an extension of noisy cross validation. SNCV can provide estimates of confidence in model predictions by assigning a quality score to each example; stratify labels to handle class imbalance; and identify likely low-quality labels to analyze the causes. We assess performance of SNCV on diagnosis of glaucoma suspect risk from retinal fundus photographs, a clinically important yet nuanced labeling task. Using training data from a previously-published deep learning model, we compute a continuous quality score (QS) for each training example. We relabel 1,277 low-QS examples using a trained glaucoma specialist; the new labels agree with the SNCV prediction over the initial label >85% of the time, indicating that low-QS examples mostly reflect labeler errors. We then quantify the impact of training with only high-QS labels, showing that strong model performance may be obtained with many fewer examples. By applying the method to randomly sub-sampled training dataset, we show that our method can reduce labelling burden by approximately 50% while achieving model performance non-inferior to using the full dataset on multiple held-out test sets.
Deep Learning to Assess Glaucoma Risk and Associated Features in Fundus Images
Dec 21, 2018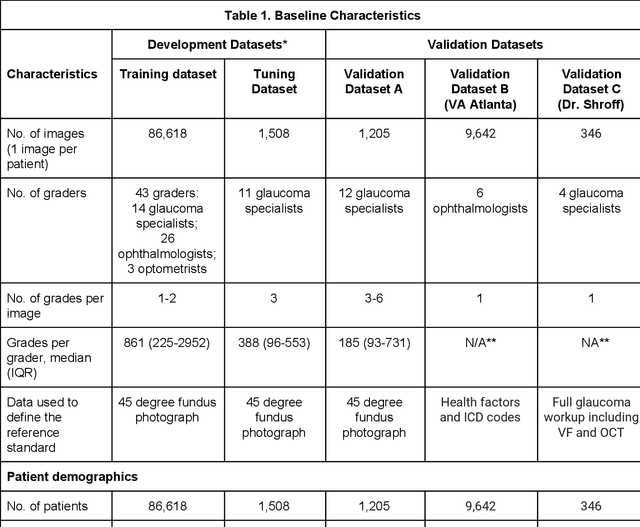
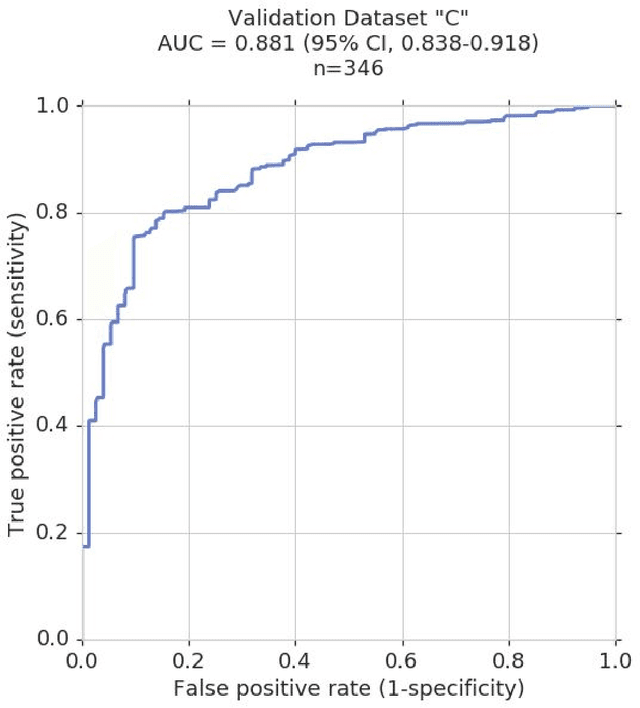
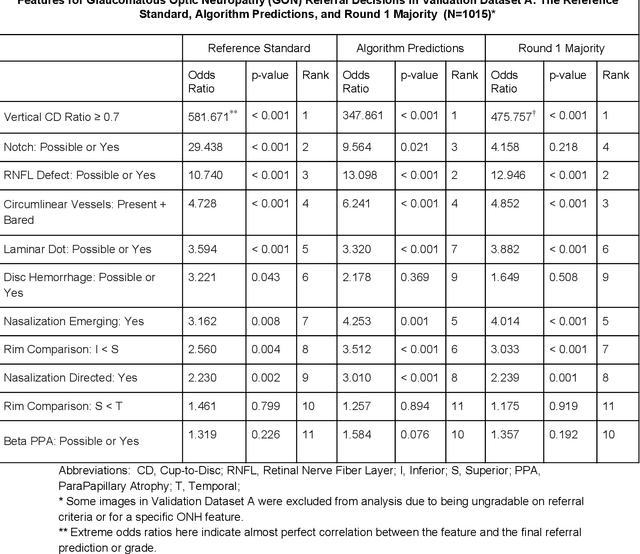
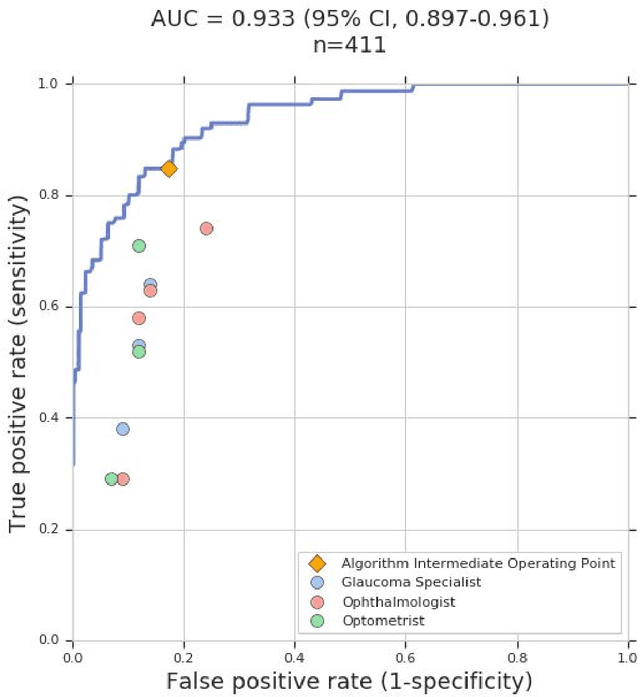
Abstract:Glaucoma is the leading cause of preventable, irreversible blindness world-wide. The disease can remain asymptomatic until severe, and an estimated 50%-90% of people with glaucoma remain undiagnosed. Thus, glaucoma screening is recommended for early detection and treatment. A cost-effective tool to detect glaucoma could expand healthcare access to a much larger patient population, but such a tool is currently unavailable. We trained a deep learning (DL) algorithm using a retrospective dataset of 58,033 images, assessed for gradability, glaucomatous optic nerve head (ONH) features, and referable glaucoma risk. The resultant algorithm was validated using 2 separate datasets. For referable glaucoma risk, the algorithm had an AUC of 0.940 (95%CI, 0.922-0.955) in validation dataset "A" (1,205 images, 1 image/patient; 19% referable where images were adjudicated by panels of fellowship-trained glaucoma specialists) and 0.858 (95% CI, 0.836-0.878) in validation dataset "B" (17,593 images from 9,643 patients; 9.2% referable where images were from the Atlanta Veterans Affairs Eye Clinic diabetic teleretinal screening program using clinical referral decisions as the reference standard). Additionally, we found that the presence of vertical cup-to-disc ratio >= 0.7, neuroretinal rim notching, retinal nerve fiber layer defect, and bared circumlinear vessels contributed most to referable glaucoma risk assessment by both glaucoma specialists and the algorithm. Algorithm AUCs ranged between 0.608-0.977 for glaucomatous ONH features. The DL algorithm was significantly more sensitive than 6 of 10 graders, including 2 of 3 glaucoma specialists, with comparable or higher specificity relative to all graders. A DL algorithm trained on fundus images alone can detect referable glaucoma risk with higher sensitivity and comparable specificity to eye care providers.
Deep Learning vs. Human Graders for Classifying Severity Levels of Diabetic Retinopathy in a Real-World Nationwide Screening Program
Oct 18, 2018
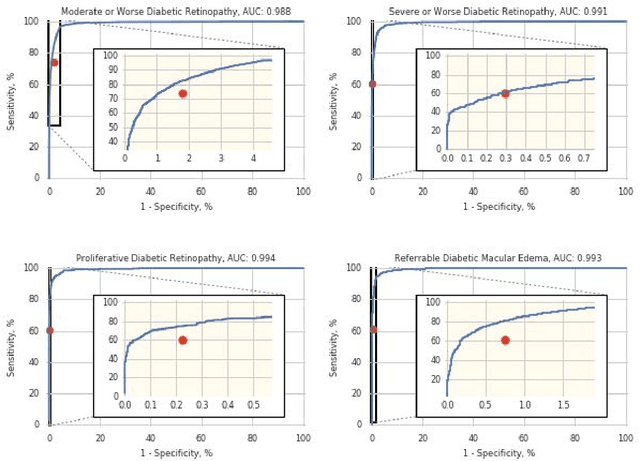
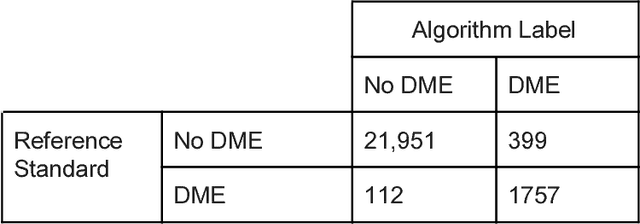
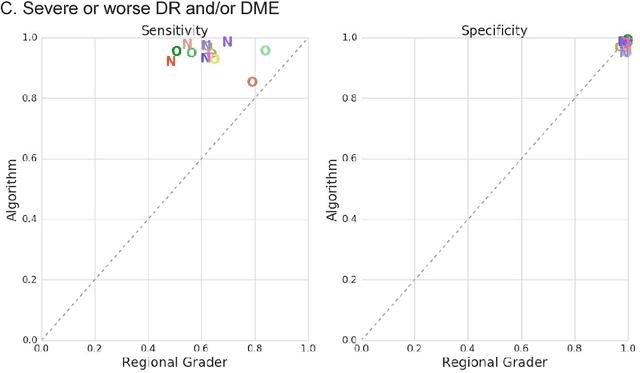
Abstract:Deep learning algorithms have been used to detect diabetic retinopathy (DR) with specialist-level accuracy. This study aims to validate one such algorithm on a large-scale clinical population, and compare the algorithm performance with that of human graders. 25,326 gradable retinal images of patients with diabetes from the community-based, nation-wide screening program of DR in Thailand were analyzed for DR severity and referable diabetic macular edema (DME). Grades adjudicated by a panel of international retinal specialists served as the reference standard. Across different severity levels of DR for determining referable disease, deep learning significantly reduced the false negative rate (by 23%) at the cost of slightly higher false positive rates (2%). Deep learning algorithms may serve as a valuable tool for DR screening.
Tool Detection and Operative Skill Assessment in Surgical Videos Using Region-Based Convolutional Neural Networks
Jul 22, 2018



Abstract:Five billion people in the world lack access to quality surgical care. Surgeon skill varies dramatically, and many surgical patients suffer complications and avoidable harm. Improving surgical training and feedback would help to reduce the rate of complications, half of which have been shown to be preventable. To do this, it is essential to assess operative skill, a process that currently requires experts and is manual, time consuming, and subjective. In this work, we introduce an approach to automatically assess surgeon performance by tracking and analyzing tool movements in surgical videos, leveraging region-based convolutional neural networks. In order to study this problem, we also introduce a new dataset, m2cai16-tool-locations, which extends the m2cai16-tool dataset with spatial bounds of tools. While previous methods have addressed tool presence detection, ours is the first to not only detect presence but also spatially localize surgical tools in real-world laparoscopic surgical videos. We show that our method both effectively detects the spatial bounds of tools as well as significantly outperforms existing methods on tool presence detection. We further demonstrate the ability of our method to assess surgical quality through analysis of tool usage patterns, movement range, and economy of motion.
Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy
Jul 03, 2018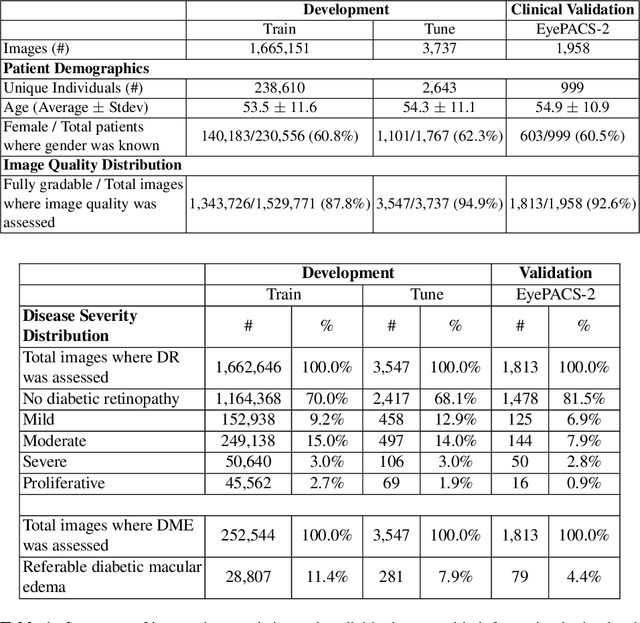
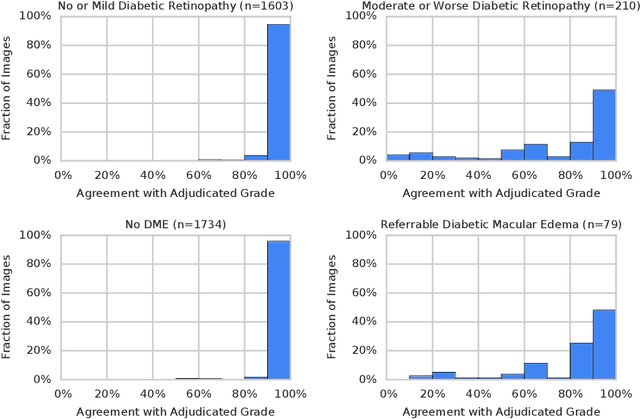
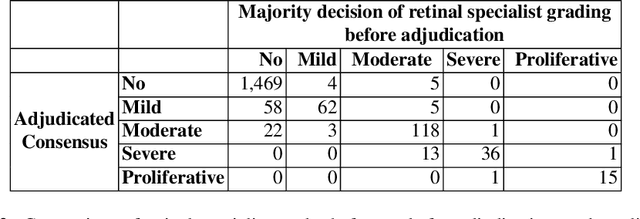
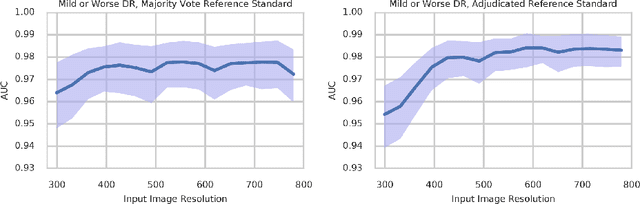
Abstract:Diabetic retinopathy (DR) and diabetic macular edema are common complications of diabetes which can lead to vision loss. The grading of DR is a fairly complex process that requires the detection of fine features such as microaneurysms, intraretinal hemorrhages, and intraretinal microvascular abnormalities. Because of this, there can be a fair amount of grader variability. There are different methods of obtaining the reference standard and resolving disagreements between graders, and while it is usually accepted that adjudication until full consensus will yield the best reference standard, the difference between various methods of resolving disagreements has not been examined extensively. In this study, we examine the variability in different methods of grading, definitions of reference standards, and their effects on building deep learning models for the detection of diabetic eye disease. We find that a small set of adjudicated DR grades allows substantial improvements in algorithm performance. The resulting algorithm's performance was on par with that of individual U.S. board-certified ophthalmologists and retinal specialists.
Scalable Annotation of Fine-Grained Categories Without Experts
Sep 07, 2017

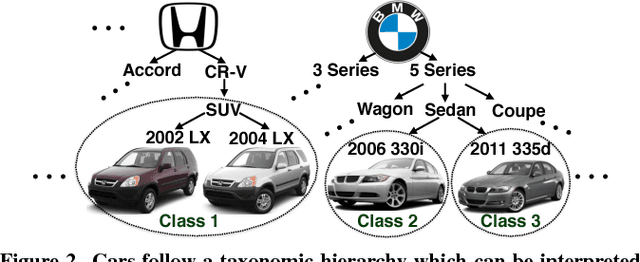

Abstract:We present a crowdsourcing workflow to collect image annotations for visually similar synthetic categories without requiring experts. In animals, there is a direct link between taxonomy and visual similarity: e.g. a collie (type of dog) looks more similar to other collies (e.g. smooth collie) than a greyhound (another type of dog). However, in synthetic categories such as cars, objects with similar taxonomy can have very different appearance: e.g. a 2011 Ford F-150 Supercrew-HD looks the same as a 2011 Ford F-150 Supercrew-LL but very different from a 2011 Ford F-150 Supercrew-SVT. We introduce a graph based crowdsourcing algorithm to automatically group visually indistinguishable objects together. Using our workflow, we label 712,430 images by ~1,000 Amazon Mechanical Turk workers; resulting in the largest fine-grained visual dataset reported to date with 2,657 categories of cars annotated at 1/20th the cost of hiring experts.
Fine-Grained Car Detection for Visual Census Estimation
Sep 07, 2017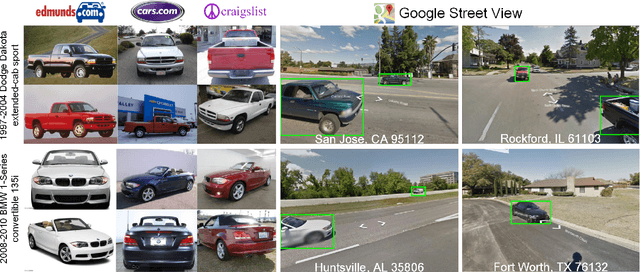
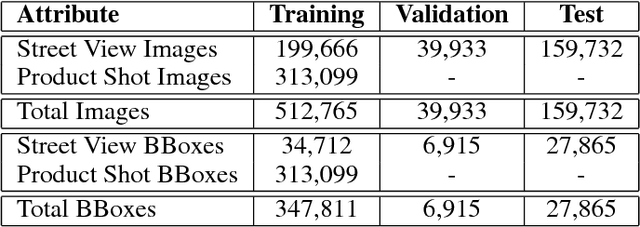
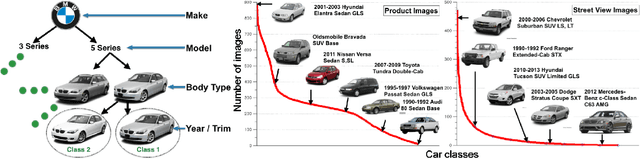
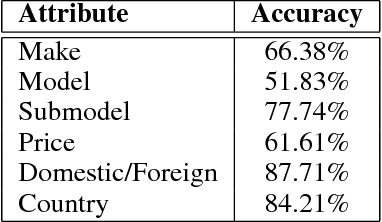
Abstract:Targeted socioeconomic policies require an accurate understanding of a country's demographic makeup. To that end, the United States spends more than 1 billion dollars a year gathering census data such as race, gender, education, occupation and unemployment rates. Compared to the traditional method of collecting surveys across many years which is costly and labor intensive, data-driven, machine learning driven approaches are cheaper and faster--with the potential ability to detect trends in close to real time. In this work, we leverage the ubiquity of Google Street View images and develop a computer vision pipeline to predict income, per capita carbon emission, crime rates and other city attributes from a single source of publicly available visual data. We first detect cars in 50 million images across 200 of the largest US cities and train a model to predict demographic attributes using the detected cars. To facilitate our work, we have collected the largest and most challenging fine-grained dataset reported to date consisting of over 2600 classes of cars comprised of images from Google Street View and other web sources, classified by car experts to account for even the most subtle of visual differences. We use this data to construct the largest scale fine-grained detection system reported to date. Our prediction results correlate well with ground truth income data (r=0.82), Massachusetts department of vehicle registration, and sources investigating crime rates, income segregation, per capita carbon emission, and other market research. Finally, we learn interesting relationships between cars and neighborhoods allowing us to perform the first large scale sociological analysis of cities using computer vision techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge