Hrvoje Bogunović
on behalf of the PINNACLE consortium
Automatic detection and prediction of nAMD activity change in retinal OCT using Siamese networks and Wasserstein Distance for ordinality
Jan 24, 2025Abstract:Neovascular age-related macular degeneration (nAMD) is a leading cause of vision loss among older adults, where disease activity detection and progression prediction are critical for nAMD management in terms of timely drug administration and improving patient outcomes. Recent advancements in deep learning offer a promising solution for predicting changes in AMD from optical coherence tomography (OCT) retinal volumes. In this work, we proposed deep learning models for the two tasks of the public MARIO Challenge at MICCAI 2024, designed to detect and forecast changes in nAMD severity with longitudinal retinal OCT. For the first task, we employ a Vision Transformer (ViT) based Siamese Network to detect changes in AMD severity by comparing scan embeddings of a patient from different time points. To train a model to forecast the change after 3 months, we exploit, for the first time, an Earth Mover (Wasserstein) Distance-based loss to harness the ordinal relation within the severity change classes. Both models ranked high on the preliminary leaderboard, demonstrating that their predictive capabilities could facilitate nAMD treatment management.
Forecasting Disease Progression with Parallel Hyperplanes in Longitudinal Retinal OCT
Sep 30, 2024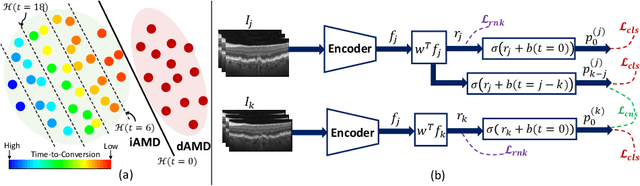
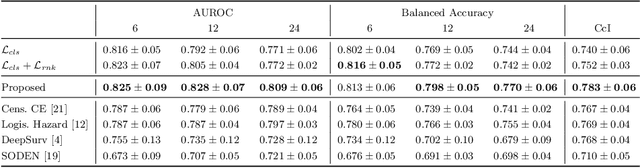
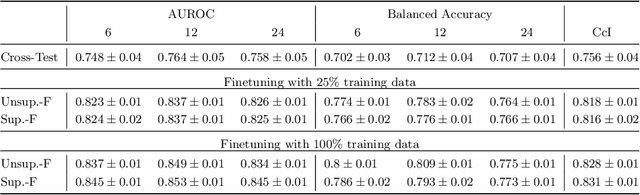
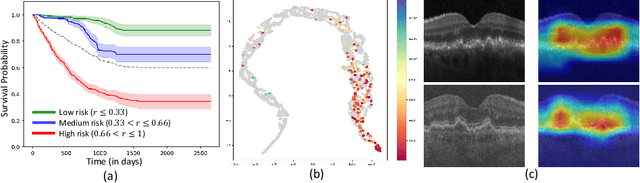
Abstract:Predicting future disease progression risk from medical images is challenging due to patient heterogeneity, and subtle or unknown imaging biomarkers. Moreover, deep learning (DL) methods for survival analysis are susceptible to image domain shifts across scanners. We tackle these issues in the task of predicting late dry Age-related Macular Degeneration (dAMD) onset from retinal OCT scans. We propose a novel DL method for survival prediction to jointly predict from the current scan a risk score, inversely related to time-to-conversion, and the probability of conversion within a time interval $t$. It uses a family of parallel hyperplanes generated by parameterizing the bias term as a function of $t$. In addition, we develop unsupervised losses based on intra-subject image pairs to ensure that risk scores increase over time and that future conversion predictions are consistent with AMD stage prediction using actual scans of future visits. Such losses enable data-efficient fine-tuning of the trained model on new unlabeled datasets acquired with a different scanner. Extensive evaluation on two large datasets acquired with different scanners resulted in a mean AUROCs of 0.82 for Dataset-1 and 0.83 for Dataset-2, across prediction intervals of 6,12 and 24 months.
Specialist vision-language models for clinical ophthalmology
Jul 11, 2024Abstract:Clinicians spend a significant amount of time reviewing medical images and transcribing their findings regarding patient diagnosis, referral and treatment in text form. Vision-language models (VLMs), which automatically interpret images and summarize their findings as text, have enormous potential to alleviate clinical workloads and increase patient access to high-quality medical care. While foundational models have stirred considerable interest in the medical community, it is unclear whether their general capabilities translate to real-world clinical utility. In this work, we show that foundation VLMs markedly underperform compared to practicing ophthalmologists on specialist tasks crucial to the care of patients with age-related macular degeneration (AMD). To address this, we initially identified the essential capabilities required for image-based clinical decision-making, and then developed a curriculum to selectively train VLMs in these skills. The resulting model, RetinaVLM, can be instructed to write reports that significantly outperform those written by leading foundation medical VLMs in disease staging (F1 score of 0.63 vs. 0.11) and patient referral (0.67 vs. 0.39), and approaches the diagnostic performance of junior ophthalmologists (who achieve 0.77 and 0.78 on the respective tasks). Furthermore, in a reader study involving two senior ophthalmologists with up to 32 years of experience, RetinaVLM's reports were found to be similarly correct (78.6% vs. 82.1%) and complete (both 78.6%) as reports written by junior ophthalmologists with up to 10 years of experience. These results demonstrate that our curriculum-based approach provides a blueprint for specializing generalist foundation medical VLMs to handle real-world clinical tasks.
Time-Equivariant Contrastive Learning for Degenerative Disease Progression in Retinal OCT
May 15, 2024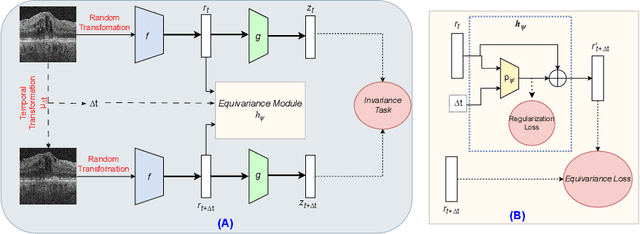
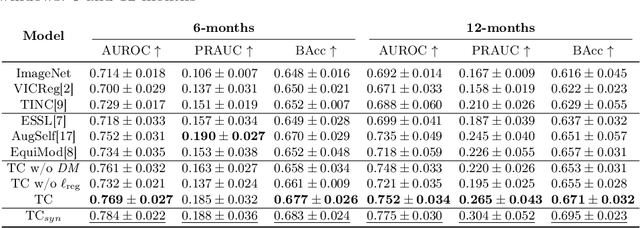
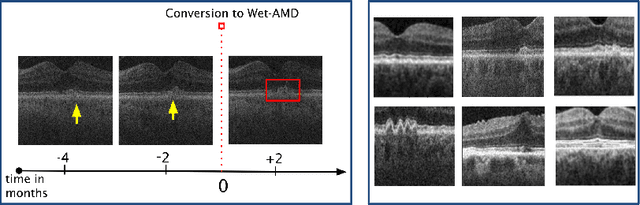
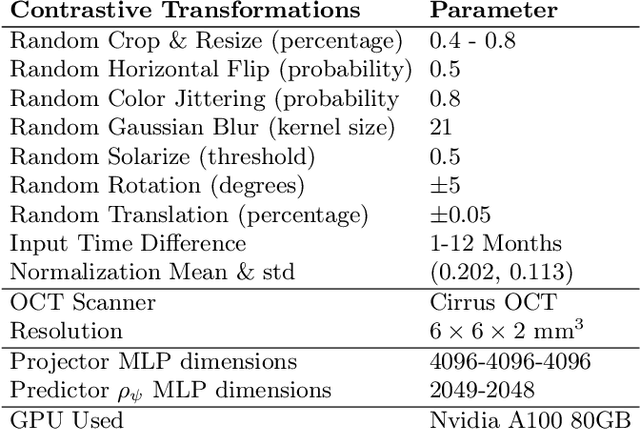
Abstract:Contrastive pretraining provides robust representations by ensuring their invariance to different image transformations while simultaneously preventing representational collapse. Equivariant contrastive learning, on the other hand, provides representations sensitive to specific image transformations while remaining invariant to others. By introducing equivariance to time-induced transformations, such as disease-related anatomical changes in longitudinal imaging, the model can effectively capture such changes in the representation space. In this work, we pro-pose a Time-equivariant Contrastive Learning (TC) method. First, an encoder embeds two unlabeled scans from different time points of the same patient into the representation space. Next, a temporal equivariance module is trained to predict the representation of a later visit based on the representation from one of the previous visits and the corresponding time interval with a novel regularization loss term while preserving the invariance property to irrelevant image transformations. On a large longitudinal dataset, our model clearly outperforms existing equivariant contrastive methods in predicting progression from intermediate age-related macular degeneration (AMD) to advanced wet-AMD within a specified time-window.
Spatiotemporal Representation Learning for Short and Long Medical Image Time Series
Mar 12, 2024Abstract:Analyzing temporal developments is crucial for the accurate prognosis of many medical conditions. Temporal changes that occur over short time scales are key to assessing the health of physiological functions, such as the cardiac cycle. Moreover, tracking longer term developments that occur over months or years in evolving processes, such as age-related macular degeneration (AMD), is essential for accurate prognosis. Despite the importance of both short and long term analysis to clinical decision making, they remain understudied in medical deep learning. State of the art methods for spatiotemporal representation learning, developed for short natural videos, prioritize the detection of temporal constants rather than temporal developments. Moreover, they do not account for varying time intervals between acquisitions, which are essential for contextualizing observed changes. To address these issues, we propose two approaches. First, we combine clip-level contrastive learning with a novel temporal embedding to adapt to irregular time series. Second, we propose masking and predicting latent frame representations of the temporal sequence. Our two approaches outperform all prior methods on temporally-dependent tasks including cardiac output estimation and three prognostic AMD tasks. Overall, this enables the automated analysis of temporal patterns which are typically overlooked in applications of deep learning to medicine.
RRWNet: Recursive Refinement Network for Effective Retinal Artery/Vein Segmentation and Classification
Feb 05, 2024



Abstract:The caliber and configuration of retinal blood vessels serve as important biomarkers for various diseases and medical conditions. A thorough analysis of the retinal vasculature requires the segmentation of blood vessels and their classification into arteries and veins, which is typically performed on color fundus images obtained by retinography, a widely used imaging technique. Nonetheless, manually performing these tasks is labor-intensive and prone to human error. Various automated methods have been proposed to address this problem. However, the current state of art in artery/vein segmentation and classification faces challenges due to manifest classification errors that affect the topological consistency of segmentation maps. This study presents an innovative end-to-end framework, RRWNet, designed to recursively refine semantic segmentation maps and correct manifest classification errors. The framework consists of a fully convolutional neural network with a Base subnetwork that generates base segmentation maps from input images, and a Recursive Refinement subnetwork that iteratively and recursively improves these maps. Evaluation on public datasets demonstrates the state-of-the-art performance of the proposed method, yielding more topologically consistent segmentation maps with fewer manifest classification errors than existing approaches. In addition, the Recursive Refinement module proves effective in post-processing segmentation maps from other methods, automatically correcting classification errors and improving topological consistency. The model code, weights, and predictions are publicly available at https://github.com/j-morano/rrwnet.
Deep Multimodal Fusion of Data with Heterogeneous Dimensionality via Projective Networks
Feb 02, 2024



Abstract:The use of multimodal imaging has led to significant improvements in the diagnosis and treatment of many diseases. Similar to clinical practice, some works have demonstrated the benefits of multimodal fusion for automatic segmentation and classification using deep learning-based methods. However, current segmentation methods are limited to fusion of modalities with the same dimensionality (e.g., 3D+3D, 2D+2D), which is not always possible, and the fusion strategies implemented by classification methods are incompatible with localization tasks. In this work, we propose a novel deep learning-based framework for the fusion of multimodal data with heterogeneous dimensionality (e.g., 3D+2D) that is compatible with localization tasks. The proposed framework extracts the features of the different modalities and projects them into the common feature subspace. The projected features are then fused and further processed to obtain the final prediction. The framework was validated on the following tasks: segmentation of geographic atrophy (GA), a late-stage manifestation of age-related macular degeneration, and segmentation of retinal blood vessels (RBV) in multimodal retinal imaging. Our results show that the proposed method outperforms the state-of-the-art monomodal methods on GA and RBV segmentation by up to 3.10% and 4.64% Dice, respectively.
3DTINC: Time-Equivariant Non-Contrastive Learning for Predicting Disease Progression from Longitudinal OCTs
Dec 28, 2023Abstract:Self-supervised learning (SSL) has emerged as a powerful technique for improving the efficiency and effectiveness of deep learning models. Contrastive methods are a prominent family of SSL that extract similar representations of two augmented views of an image while pushing away others in the representation space as negatives. However, the state-of-the-art contrastive methods require large batch sizes and augmentations designed for natural images that are impractical for 3D medical images. To address these limitations, we propose a new longitudinal SSL method, 3DTINC, based on non-contrastive learning. It is designed to learn perturbation-invariant features for 3D optical coherence tomography (OCT) volumes, using augmentations specifically designed for OCT. We introduce a new non-contrastive similarity loss term that learns temporal information implicitly from intra-patient scans acquired at different times. Our experiments show that this temporal information is crucial for predicting progression of retinal diseases, such as age-related macular degeneration (AMD). After pretraining with 3DTINC, we evaluated the learned representations and the prognostic models on two large-scale longitudinal datasets of retinal OCTs where we predict the conversion to wet-AMD within a six months interval. Our results demonstrate that each component of our contributions is crucial for learning meaningful representations useful in predicting disease progression from longitudinal volumetric scans.
SAMedOCT: Adapting Segment Anything Model (SAM) for Retinal OCT
Aug 31, 2023Abstract:The Segment Anything Model (SAM) has gained significant attention in the field of image segmentation due to its impressive capabilities and prompt-based interface. While SAM has already been extensively evaluated in various domains, its adaptation to retinal OCT scans remains unexplored. To bridge this research gap, we conduct a comprehensive evaluation of SAM and its adaptations on a large-scale public dataset of OCTs from RETOUCH challenge. Our evaluation covers diverse retinal diseases, fluid compartments, and device vendors, comparing SAM against state-of-the-art retinal fluid segmentation methods. Through our analysis, we showcase adapted SAM's efficacy as a powerful segmentation model in retinal OCT scans, although still lagging behind established methods in some circumstances. The findings highlight SAM's adaptability and robustness, showcasing its utility as a valuable tool in retinal OCT image analysis and paving the way for further advancements in this domain.
Pretrained Deep 2.5D Models for Efficient Predictive Modeling from Retinal OCT
Jul 25, 2023Abstract:In the field of medical imaging, 3D deep learning models play a crucial role in building powerful predictive models of disease progression. However, the size of these models presents significant challenges, both in terms of computational resources and data requirements. Moreover, achieving high-quality pretraining of 3D models proves to be even more challenging. To address these issues, hybrid 2.5D approaches provide an effective solution for utilizing 3D volumetric data efficiently using 2D models. Combining 2D and 3D techniques offers a promising avenue for optimizing performance while minimizing memory requirements. In this paper, we explore 2.5D architectures based on a combination of convolutional neural networks (CNNs), long short-term memory (LSTM), and Transformers. In addition, leveraging the benefits of recent non-contrastive pretraining approaches in 2D, we enhanced the performance and data efficiency of 2.5D techniques even further. We demonstrate the effectiveness of architectures and associated pretraining on a task of predicting progression to wet age-related macular degeneration (AMD) within a six-month period on two large longitudinal OCT datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge