Botond Fazekas
MIRAGE: Multimodal foundation model and benchmark for comprehensive retinal OCT image analysis
Jun 11, 2025Abstract:Artificial intelligence (AI) has become a fundamental tool for assisting clinicians in analyzing ophthalmic images, such as optical coherence tomography (OCT). However, developing AI models often requires extensive annotation, and existing models tend to underperform on independent, unseen data. Foundation models (FMs), large AI models trained on vast unlabeled datasets, have shown promise in overcoming these challenges. Nonetheless, available FMs for ophthalmology lack extensive validation, especially for segmentation tasks, and focus on a single imaging modality. In this context, we propose MIRAGE, a novel multimodal FM for the analysis of OCT and scanning laser ophthalmoscopy (SLO) images. Additionally, we propose a new evaluation benchmark with OCT/SLO classification and segmentation tasks. The comparison with general and specialized FMs and segmentation methods shows the superiority of MIRAGE in both types of tasks, highlighting its suitability as a basis for the development of robust AI systems for retinal OCT image analysis. Both MIRAGE and the evaluation benchmark are publicly available: https://github.com/j-morano/MIRAGE.
SAMedOCT: Adapting Segment Anything Model (SAM) for Retinal OCT
Aug 31, 2023Abstract:The Segment Anything Model (SAM) has gained significant attention in the field of image segmentation due to its impressive capabilities and prompt-based interface. While SAM has already been extensively evaluated in various domains, its adaptation to retinal OCT scans remains unexplored. To bridge this research gap, we conduct a comprehensive evaluation of SAM and its adaptations on a large-scale public dataset of OCTs from RETOUCH challenge. Our evaluation covers diverse retinal diseases, fluid compartments, and device vendors, comparing SAM against state-of-the-art retinal fluid segmentation methods. Through our analysis, we showcase adapted SAM's efficacy as a powerful segmentation model in retinal OCT scans, although still lagging behind established methods in some circumstances. The findings highlight SAM's adaptability and robustness, showcasing its utility as a valuable tool in retinal OCT image analysis and paving the way for further advancements in this domain.
Segmentation of Bruch's Membrane in retinal OCT with AMD using anatomical priors and uncertainty quantification
Oct 30, 2022Abstract:Bruch's membrane (BM) segmentation on optical coherence tomography (OCT) is a pivotal step for the diagnosis and follow-up of age-related macular degeneration (AMD), one of the leading causes of blindness in the developed world. Automated BM segmentation methods exist, but they usually do not account for the anatomical coherence of the results, neither provide feedback on the confidence of the prediction. These factors limit the applicability of these systems in real-world scenarios. With this in mind, we propose an end-to-end deep learning method for automated BM segmentation in AMD patients. An Attention U-Net is trained to output a probability density function of the BM position, while taking into account the natural curvature of the surface. Besides the surface position, the method also estimates an A-scan wise uncertainty measure of the segmentation output. Subsequently, the A-scans with high uncertainty are interpolated using thin plate splines (TPS). We tested our method with ablation studies on an internal dataset with 138 patients covering all three AMD stages, and achieved a mean absolute localization error of 4.10 um. In addition, the proposed segmentation method was compared against the state-of-the-art methods and showed a superior performance on an external publicly available dataset from a different patient cohort and OCT device, demonstrating strong generalization ability.
SD-LayerNet: Semi-supervised retinal layer segmentation in OCT using disentangled representation with anatomical priors
Jul 01, 2022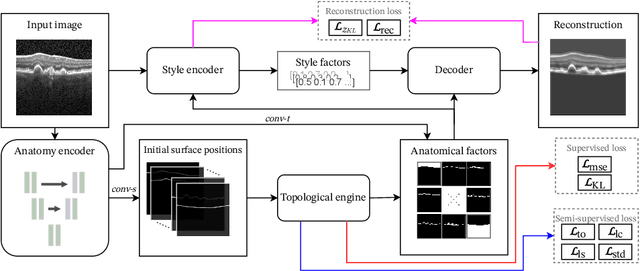
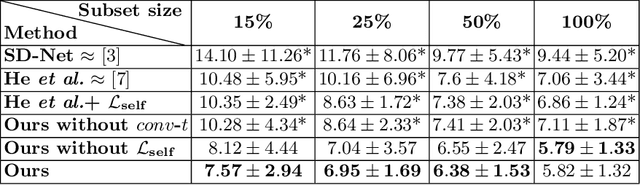
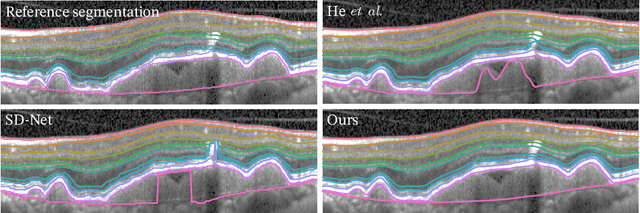
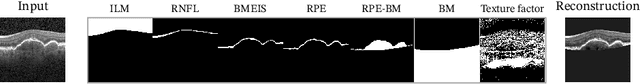
Abstract:Optical coherence tomography (OCT) is a non-invasive 3D modality widely used in ophthalmology for imaging the retina. Achieving automated, anatomically coherent retinal layer segmentation on OCT is important for the detection and monitoring of different retinal diseases, like Age-related Macular Disease (AMD) or Diabetic Retinopathy. However, the majority of state-of-the-art layer segmentation methods are based on purely supervised deep-learning, requiring a large amount of pixel-level annotated data that is expensive and hard to obtain. With this in mind, we introduce a semi-supervised paradigm into the retinal layer segmentation task that makes use of the information present in large-scale unlabeled datasets as well as anatomical priors. In particular, a novel fully differentiable approach is used for converting surface position regression into a pixel-wise structured segmentation, allowing to use both 1D surface and 2D layer representations in a coupled fashion to train the model. In particular, these 2D segmentations are used as anatomical factors that, together with learned style factors, compose disentangled representations used for reconstructing the input image. In parallel, we propose a set of anatomical priors to improve network training when a limited amount of labeled data is available. We demonstrate on the real-world dataset of scans with intermediate and wet-AMD that our method outperforms state-of-the-art when using our full training set, but more importantly largely exceeds state-of-the-art when it is trained with a fraction of the labeled data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge