Haikun Qi
Coordinate-Based Neural Representation Enabling Zero-Shot Learning for 3D Multiparametric Quantitative MRI
Oct 02, 2024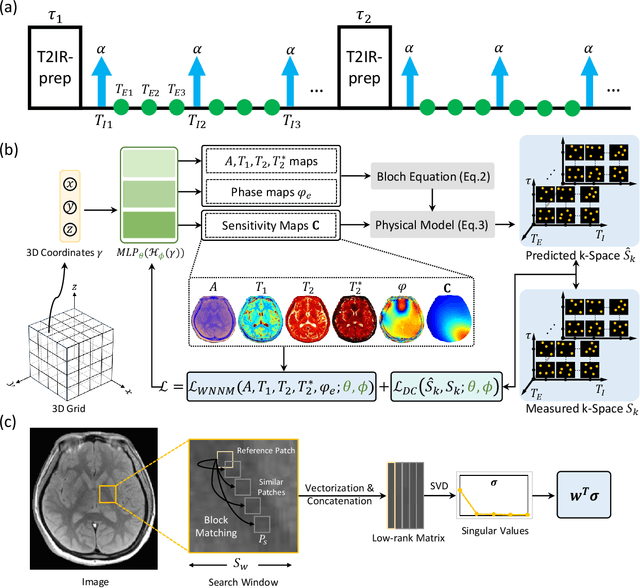

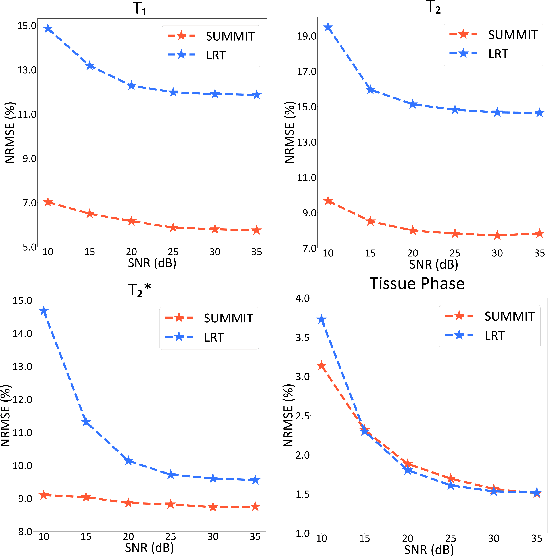
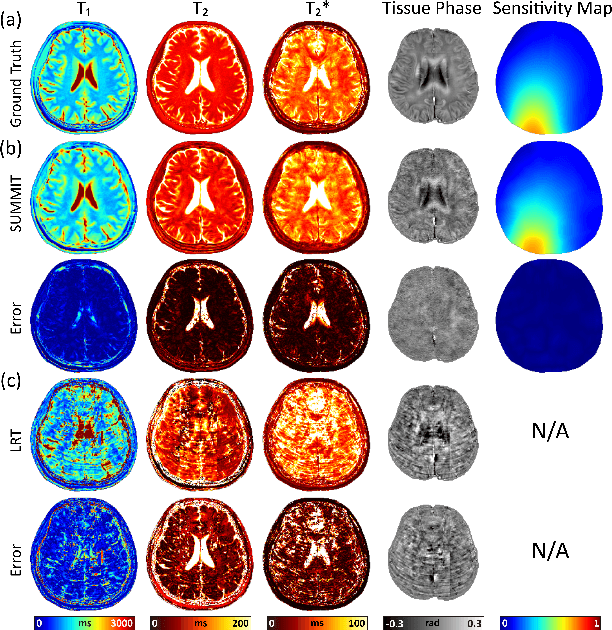
Abstract:Quantitative magnetic resonance imaging (qMRI) offers tissue-specific physical parameters with significant potential for neuroscience research and clinical practice. However, lengthy scan times for 3D multiparametric qMRI acquisition limit its clinical utility. Here, we propose SUMMIT, an innovative imaging methodology that includes data acquisition and an unsupervised reconstruction for simultaneous multiparametric qMRI. SUMMIT first encodes multiple important quantitative properties into highly undersampled k-space. It further leverages implicit neural representation incorporated with a dedicated physics model to reconstruct the desired multiparametric maps without needing external training datasets. SUMMIT delivers co-registered T1, T2, T2*, and quantitative susceptibility mapping. Extensive simulations and phantom imaging demonstrate SUMMIT's high accuracy. Additionally, the proposed unsupervised approach for qMRI reconstruction also introduces a novel zero-shot learning paradigm for multiparametric imaging applicable to various medical imaging modalities.
A Survey of Emerging Applications of Diffusion Probabilistic Models in MRI
Nov 19, 2023



Abstract:Diffusion probabilistic models (DPMs) which employ explicit likelihood characterization and a gradual sampling process to synthesize data, have gained increasing research interest. Despite their huge computational burdens due to the large number of steps involved during sampling, DPMs are widely appreciated in various medical imaging tasks for their high-quality and diversity of generation. Magnetic resonance imaging (MRI) is an important medical imaging modality with excellent soft tissue contrast and superb spatial resolution, which possesses unique opportunities for diffusion models. Although there is a recent surge of studies exploring DPMs in MRI, a survey paper of DPMs specifically designed for MRI applications is still lacking. This review article aims to help researchers in the MRI community to grasp the advances of DPMs in different applications. We first introduce the theory of two dominant kinds of DPMs, categorized according to whether the diffusion time step is discrete or continuous, and then provide a comprehensive review of emerging DPMs in MRI, including reconstruction, image generation, image translation, segmentation, anomaly detection, and further research topics. Finally, we discuss the general limitations as well as limitations specific to the MRI tasks of DPMs and point out potential areas that are worth further exploration.
Fast T2w/FLAIR MRI Acquisition by Optimal Sampling of Information Complementary to Pre-acquired T1w MRI
Nov 11, 2021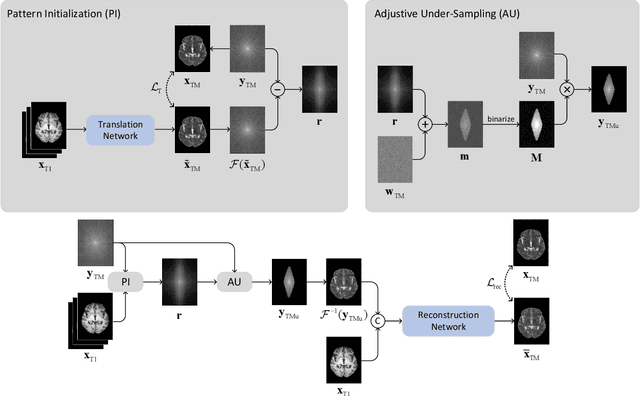
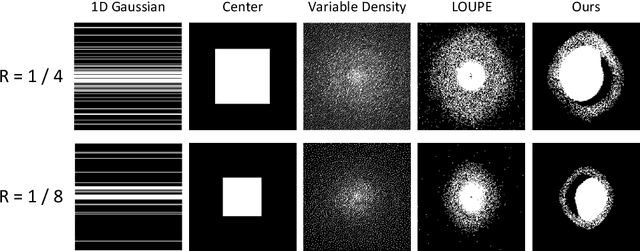

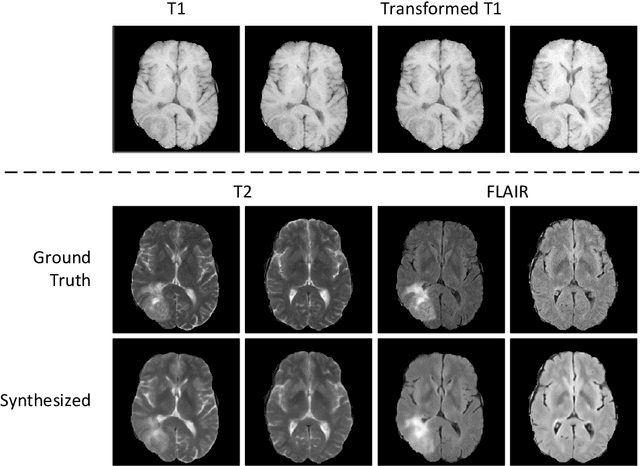
Abstract:Recent studies on T1-assisted MRI reconstruction for under-sampled images of other modalities have demonstrated the potential of further accelerating MRI acquisition of other modalities. Most of the state-of-the-art approaches have achieved improvement through the development of network architectures for fixed under-sampling patterns, without fully exploiting the complementary information between modalities. Although existing under-sampling pattern learning algorithms can be simply modified to allow the fully-sampled T1-weighted MR image to assist the pattern learning, no significant improvement on the reconstruction task can be achieved. To this end, we propose an iterative framework to optimize the under-sampling pattern for MRI acquisition of another modality that can complement the fully-sampled T1-weighted MR image at different under-sampling factors, while jointly optimizing the T1-assisted MRI reconstruction model. Specifically, our proposed method exploits the difference of latent information between the two modalities for determining the sampling patterns that can maximize the assistance power of T1-weighted MR image in improving the MRI reconstruction. We have demonstrated superior performance of our learned under-sampling patterns on a public dataset, compared to commonly used under-sampling patterns and state-of-the-art methods that can jointly optimize both the reconstruction network and the under-sampling pattern, up to 8-fold under-sampling factor.
LAPNet: Non-rigid Registration derived in k-space for Magnetic Resonance Imaging
Jul 19, 2021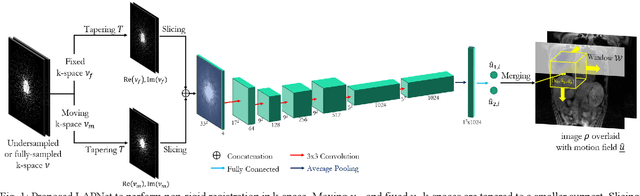

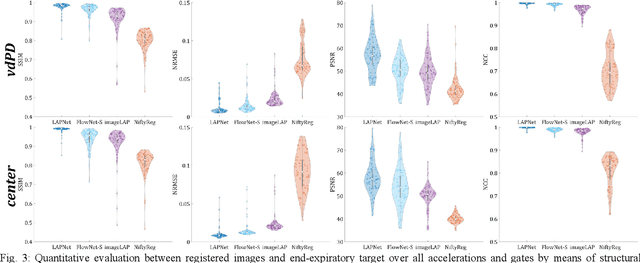
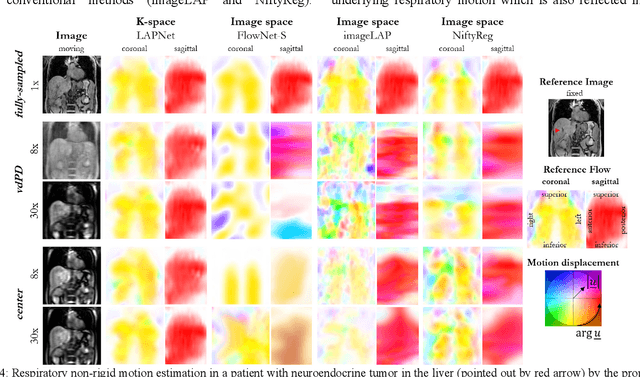
Abstract:Physiological motion, such as cardiac and respiratory motion, during Magnetic Resonance (MR) image acquisition can cause image artifacts. Motion correction techniques have been proposed to compensate for these types of motion during thoracic scans, relying on accurate motion estimation from undersampled motion-resolved reconstruction. A particular interest and challenge lie in the derivation of reliable non-rigid motion fields from the undersampled motion-resolved data. Motion estimation is usually formulated in image space via diffusion, parametric-spline, or optical flow methods. However, image-based registration can be impaired by remaining aliasing artifacts due to the undersampled motion-resolved reconstruction. In this work, we describe a formalism to perform non-rigid registration directly in the sampled Fourier space, i.e. k-space. We propose a deep-learning based approach to perform fast and accurate non-rigid registration from the undersampled k-space data. The basic working principle originates from the Local All-Pass (LAP) technique, a recently introduced optical flow-based registration. The proposed LAPNet is compared against traditional and deep learning image-based registrations and tested on fully-sampled and highly-accelerated (with two undersampling strategies) 3D respiratory motion-resolved MR images in a cohort of 40 patients with suspected liver or lung metastases and 25 healthy subjects. The proposed LAPNet provided consistent and superior performance to image-based approaches throughout different sampling trajectories and acceleration factors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge