Ruimin Feng
OmniMRI: A Unified Vision--Language Foundation Model for Generalist MRI Interpretation
Aug 24, 2025Abstract:Magnetic Resonance Imaging (MRI) is indispensable in clinical practice but remains constrained by fragmented, multi-stage workflows encompassing acquisition, reconstruction, segmentation, detection, diagnosis, and reporting. While deep learning has achieved progress in individual tasks, existing approaches are often anatomy- or application-specific and lack generalizability across diverse clinical settings. Moreover, current pipelines rarely integrate imaging data with complementary language information that radiologists rely on in routine practice. Here, we introduce OmniMRI, a unified vision-language foundation model designed to generalize across the entire MRI workflow. OmniMRI is trained on a large-scale, heterogeneous corpus curated from 60 public datasets, over 220,000 MRI volumes and 19 million MRI slices, incorporating image-only data, paired vision-text data, and instruction-response data. Its multi-stage training paradigm, comprising self-supervised vision pretraining, vision-language alignment, multimodal pretraining, and multi-task instruction tuning, progressively equips the model with transferable visual representations, cross-modal reasoning, and robust instruction-following capabilities. Qualitative results demonstrate OmniMRI's ability to perform diverse tasks within a single architecture, including MRI reconstruction, anatomical and pathological segmentation, abnormality detection, diagnostic suggestion, and radiology report generation. These findings highlight OmniMRI's potential to consolidate fragmented pipelines into a scalable, generalist framework, paving the way toward foundation models that unify imaging and clinical language for comprehensive, end-to-end MRI interpretation.
Coordinate-Based Neural Representation Enabling Zero-Shot Learning for 3D Multiparametric Quantitative MRI
Oct 02, 2024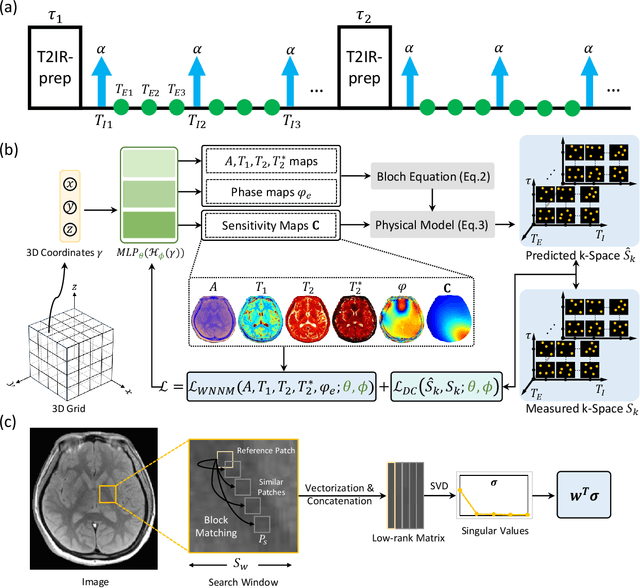

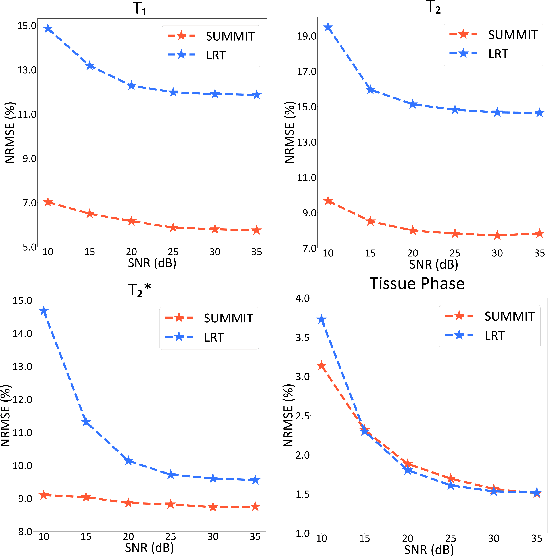
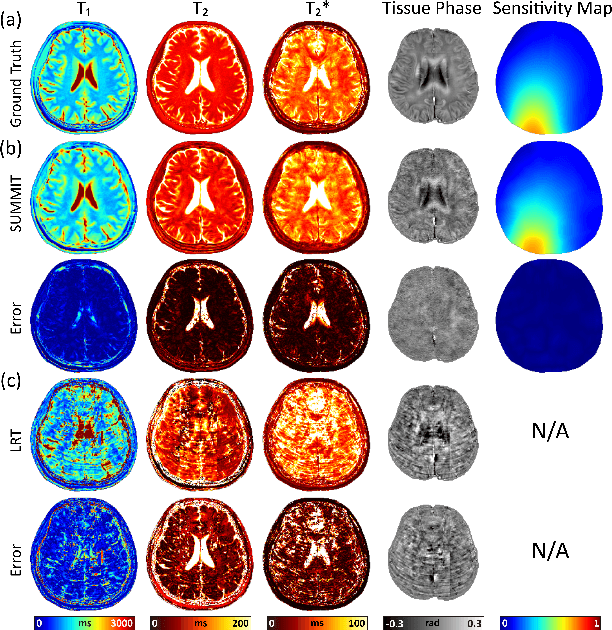
Abstract:Quantitative magnetic resonance imaging (qMRI) offers tissue-specific physical parameters with significant potential for neuroscience research and clinical practice. However, lengthy scan times for 3D multiparametric qMRI acquisition limit its clinical utility. Here, we propose SUMMIT, an innovative imaging methodology that includes data acquisition and an unsupervised reconstruction for simultaneous multiparametric qMRI. SUMMIT first encodes multiple important quantitative properties into highly undersampled k-space. It further leverages implicit neural representation incorporated with a dedicated physics model to reconstruct the desired multiparametric maps without needing external training datasets. SUMMIT delivers co-registered T1, T2, T2*, and quantitative susceptibility mapping. Extensive simulations and phantom imaging demonstrate SUMMIT's high accuracy. Additionally, the proposed unsupervised approach for qMRI reconstruction also introduces a novel zero-shot learning paradigm for multiparametric imaging applicable to various medical imaging modalities.
IMJENSE: Scan-specific Implicit Representation for Joint Coil Sensitivity and Image Estimation in Parallel MRI
Nov 21, 2023



Abstract:Parallel imaging is a commonly used technique to accelerate magnetic resonance imaging (MRI) data acquisition. Mathematically, parallel MRI reconstruction can be formulated as an inverse problem relating the sparsely sampled k-space measurements to the desired MRI image. Despite the success of many existing reconstruction algorithms, it remains a challenge to reliably reconstruct a high-quality image from highly reduced k-space measurements. Recently, implicit neural representation has emerged as a powerful paradigm to exploit the internal information and the physics of partially acquired data to generate the desired object. In this study, we introduced IMJENSE, a scan-specific implicit neural representation-based method for improving parallel MRI reconstruction. Specifically, the underlying MRI image and coil sensitivities were modeled as continuous functions of spatial coordinates, parameterized by neural networks and polynomials, respectively. The weights in the networks and coefficients in the polynomials were simultaneously learned directly from sparsely acquired k-space measurements, without fully sampled ground truth data for training. Benefiting from the powerful continuous representation and joint estimation of the MRI image and coil sensitivities, IMJENSE outperforms conventional image or k-space domain reconstruction algorithms. With extremely limited calibration data, IMJENSE is more stable than supervised calibrationless and calibration-based deep-learning methods. Results show that IMJENSE robustly reconstructs the images acquired at 5$\mathbf{\times}$ and 6$\mathbf{\times}$ accelerations with only 4 or 8 calibration lines in 2D Cartesian acquisitions, corresponding to 22.0% and 19.5% undersampling rates. The high-quality results and scanning specificity make the proposed method hold the potential for further accelerating the data acquisition of parallel MRI.
JSMoCo: Joint Coil Sensitivity and Motion Correction in Parallel MRI with a Self-Calibrating Score-Based Diffusion Model
Oct 14, 2023



Abstract:Magnetic Resonance Imaging (MRI) stands as a powerful modality in clinical diagnosis. However, it is known that MRI faces challenges such as long acquisition time and vulnerability to motion-induced artifacts. Despite the success of many existing motion correction algorithms, there has been limited research focused on correcting motion artifacts on the estimated coil sensitivity maps for fast MRI reconstruction. Existing methods might suffer from severe performance degradation due to error propagation resulting from the inaccurate coil sensitivity maps estimation. In this work, we propose to jointly estimate the motion parameters and coil sensitivity maps for under-sampled MRI reconstruction, referred to as JSMoCo. However, joint estimation of motion parameters and coil sensitivities results in a highly ill-posed inverse problem due to an increased number of unknowns. To address this, we introduce score-based diffusion models as powerful priors and leverage the MRI physical principles to efficiently constrain the solution space for this optimization problem. Specifically, we parameterize the rigid motion as three trainable variables and model coil sensitivity maps as polynomial functions. Leveraging the physical knowledge, we then employ Gibbs sampler for joint estimation, ensuring system consistency between sensitivity maps and desired images, avoiding error propagation from pre-estimated sensitivity maps to the reconstructed images. We conduct comprehensive experiments to evaluate the performance of JSMoCo on the fastMRI dataset. The results show that our method is capable of reconstructing high-quality MRI images from sparsely-sampled k-space data, even affected by motion. It achieves this by accurately estimating both motion parameters and coil sensitivities, effectively mitigating motion-related challenges during MRI reconstruction.
Spatiotemporal implicit neural representation for unsupervised dynamic MRI reconstruction
Dec 31, 2022


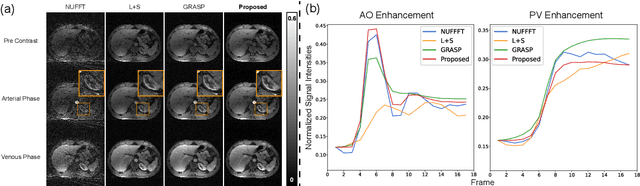
Abstract:Supervised Deep-Learning (DL)-based reconstruction algorithms have shown state-of-the-art results for highly-undersampled dynamic Magnetic Resonance Imaging (MRI) reconstruction. However, the requirement of excessive high-quality ground-truth data hinders their applications due to the generalization problem. Recently, Implicit Neural Representation (INR) has appeared as a powerful DL-based tool for solving the inverse problem by characterizing the attributes of a signal as a continuous function of corresponding coordinates in an unsupervised manner. In this work, we proposed an INR-based method to improve dynamic MRI reconstruction from highly undersampled k-space data, which only takes spatiotemporal coordinates as inputs. Specifically, the proposed INR represents the dynamic MRI images as an implicit function and encodes them into neural networks. The weights of the network are learned from sparsely-acquired (k, t)-space data itself only, without external training datasets or prior images. Benefiting from the strong implicit continuity regularization of INR together with explicit regularization for low-rankness and sparsity, our proposed method outperforms the compared scan-specific methods at various acceleration factors. E.g., experiments on retrospective cardiac cine datasets show an improvement of 5.5 ~ 7.1 dB in PSNR for extremely high accelerations (up to 41.6-fold). The high-quality and inner continuity of the images provided by INR has great potential to further improve the spatiotemporal resolution of dynamic MRI, without the need of any training data.
A scan-specific unsupervised method for parallel MRI reconstruction via implicit neural representation
Oct 19, 2022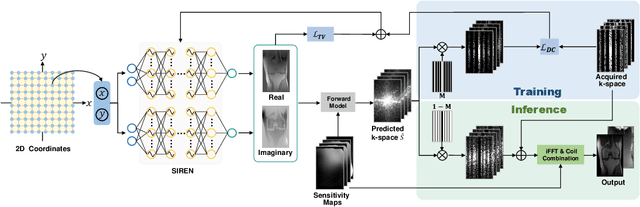
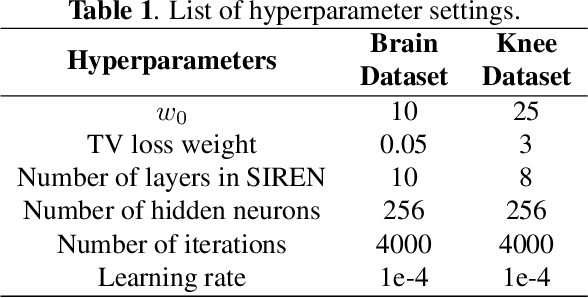
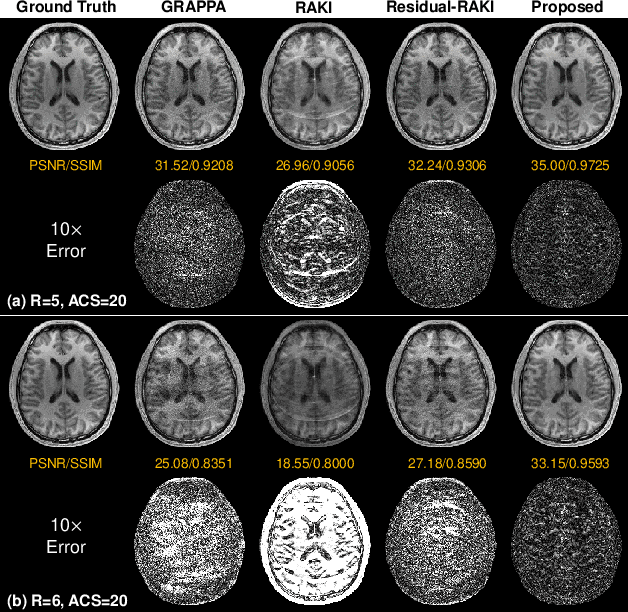
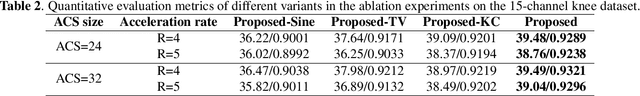
Abstract:Parallel imaging is a widely-used technique to accelerate magnetic resonance imaging (MRI). However, current methods still perform poorly in reconstructing artifact-free MRI images from highly undersampled k-space data. Recently, implicit neural representation (INR) has emerged as a new deep learning paradigm for learning the internal continuity of an object. In this study, we adopted INR to parallel MRI reconstruction. The MRI image was modeled as a continuous function of spatial coordinates. This function was parameterized by a neural network and learned directly from the measured k-space itself without additional fully sampled high-quality training data. Benefitting from the powerful continuous representations provided by INR, the proposed method outperforms existing methods by suppressing the aliasing artifacts and noise, especially at higher acceleration rates and smaller sizes of the auto-calibration signals. The high-quality results and scanning specificity make the proposed method hold the potential for further accelerating the data acquisition of parallel MRI.
Self-Supervised Coordinate Projection Network for Sparse-View Computed Tomography
Sep 12, 2022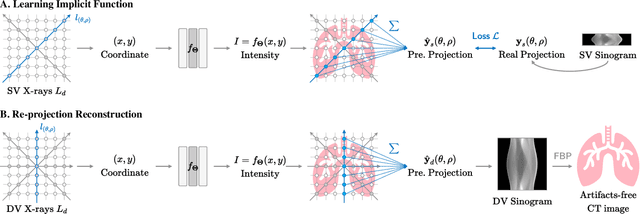
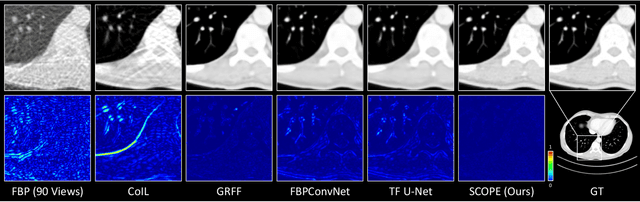
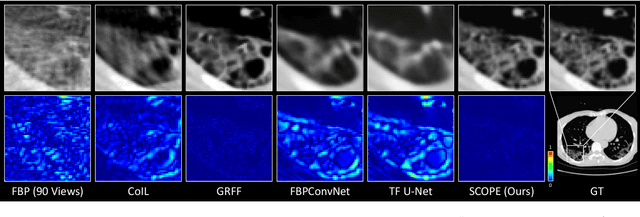
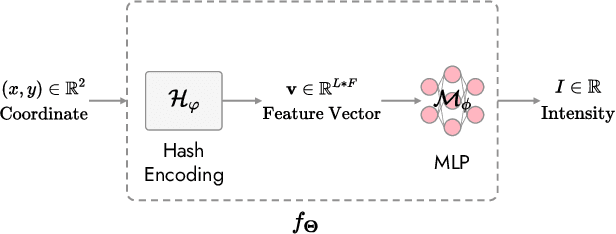
Abstract:In the present work, we propose a Self-supervised COordinate Projection nEtwork (SCOPE) to reconstruct the artifacts-free CT image from a single SV sinogram by solving the inverse tomography imaging problem. Compared with recent related works that solve similar problems using implicit neural representation network (INR), our essential contribution is an effective and simple re-projection strategy that pushes the tomography image reconstruction quality over supervised deep learning CT reconstruction works. The proposed strategy is inspired by the simple relationship between linear algebra and inverse problems. To solve the under-determined linear equation system, we first introduce INR to constrain the solution space via image continuity prior and achieve a rough solution. And secondly, we propose to generate a dense view sinogram that improves the rank of the linear equation system and produces a more stable CT image solution space. Our experiment results demonstrate that the re-projection strategy significantly improves the image reconstruction quality (+3 dB for PSNR at least). Besides, we integrate the recent hash encoding into our SCOPE model, which greatly accelerates the model training. Finally, we evaluate SCOPE in parallel and fan X-ray beam SVCT reconstruction tasks. Experimental results indicate that the proposed SCOPE model outperforms two latest INR-based methods and two well-popular supervised DL methods quantitatively and qualitatively.
MoG-QSM: Model-based Generative Adversarial Deep Learning Network for Quantitative Susceptibility Mapping
Jan 21, 2021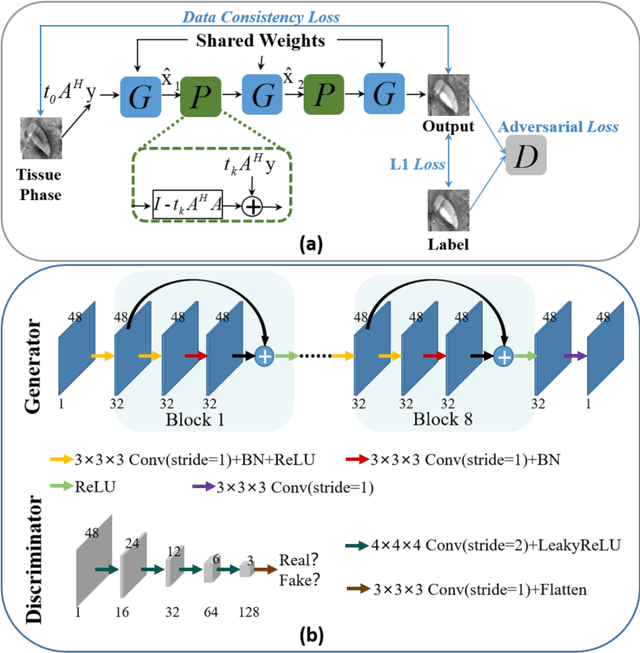
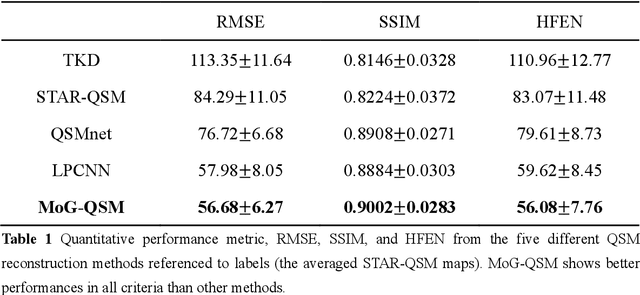
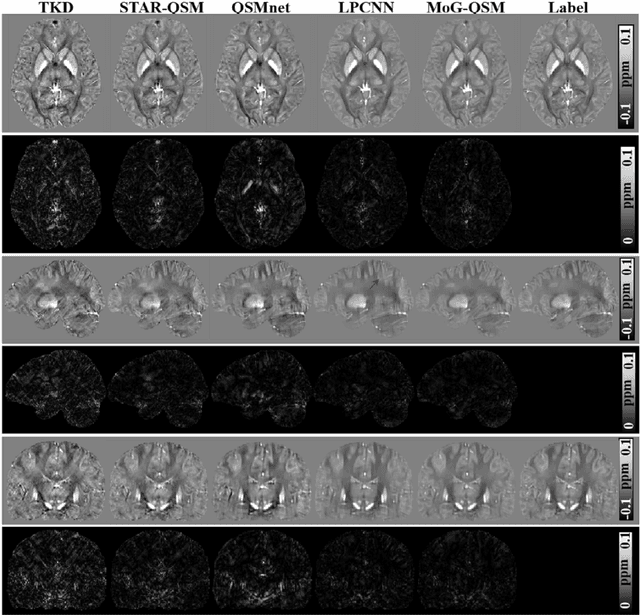
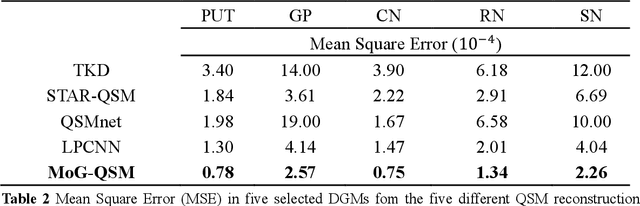
Abstract:Quantitative susceptibility mapping (QSM) estimates the underlying tissue magnetic susceptibility from the MRI gradient-echo phase signal and has demonstrated great potential in quantifying tissue susceptibility in various brain diseases. However, the intrinsic ill-posed inverse problem relating the tissue phase to the underlying susceptibility distribution affects the accuracy for quantifying tissue susceptibility. The resulting susceptibility map is known to suffer from noise amplification and streaking artifacts. To address these challenges, we propose a model-based framework that permeates benefits from generative adversarial networks to train a regularization term that contains prior information to constrain the solution of the inverse problem, referred to as MoG-QSM. A residual network leveraging a mixture of least-squares (LS) GAN and the L1 cost was trained as the generator to learn the prior information in susceptibility maps. A multilayer convolutional neural network was jointly trained to discriminate the quality of output images. MoG-QSM generates highly accurate susceptibility maps from single orientation phase maps. Quantitative evaluation parameters were compared with recently developed deep learning QSM methods and the results showed MoG-QSM achieves the best performance. Furthermore, a higher intraclass correlation coefficient (ICC) was obtained from MoG-QSM maps of the traveling subjects, demonstrating its potential for future applications, such as large cohorts of multi-center studies. MoG-QSM is also helpful for reliable longitudinal measurement of susceptibility time courses, enabling more precise monitoring for metal ion accumulation in neurodegenerative disorders.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge