René Botnar
Unsupervised reconstruction of accelerated cardiac cine MRI using Neural Fields
Jul 24, 2023



Abstract:Cardiac cine MRI is the gold standard for cardiac functional assessment, but the inherently slow acquisition process creates the necessity of reconstruction approaches for accelerated undersampled acquisitions. Several regularization approaches that exploit spatial-temporal redundancy have been proposed to reconstruct undersampled cardiac cine MRI. More recently, methods based on supervised deep learning have been also proposed to further accelerate acquisition and reconstruction. However, these techniques rely on usually large dataset for training, which are not always available. In this work, we propose an unsupervised approach based on implicit neural field representations for cardiac cine MRI (so called NF-cMRI). The proposed method was evaluated in in-vivo undersampled golden-angle radial multi-coil acquisitions for undersampling factors of 26x and 52x, achieving good image quality, and comparable spatial and improved temporal depiction than a state-of-the-art reconstruction technique.
LAPNet: Non-rigid Registration derived in k-space for Magnetic Resonance Imaging
Jul 19, 2021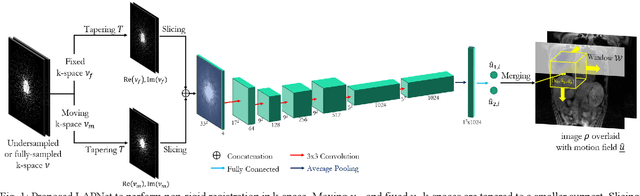

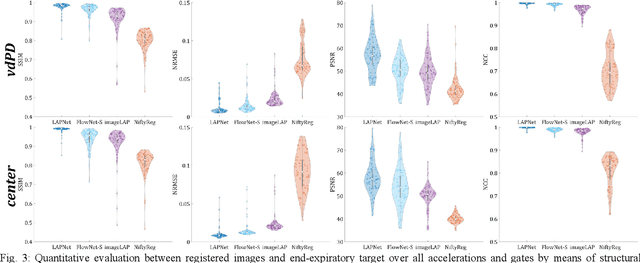
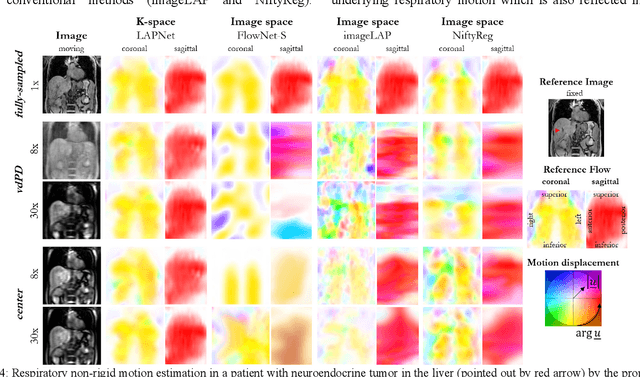
Abstract:Physiological motion, such as cardiac and respiratory motion, during Magnetic Resonance (MR) image acquisition can cause image artifacts. Motion correction techniques have been proposed to compensate for these types of motion during thoracic scans, relying on accurate motion estimation from undersampled motion-resolved reconstruction. A particular interest and challenge lie in the derivation of reliable non-rigid motion fields from the undersampled motion-resolved data. Motion estimation is usually formulated in image space via diffusion, parametric-spline, or optical flow methods. However, image-based registration can be impaired by remaining aliasing artifacts due to the undersampled motion-resolved reconstruction. In this work, we describe a formalism to perform non-rigid registration directly in the sampled Fourier space, i.e. k-space. We propose a deep-learning based approach to perform fast and accurate non-rigid registration from the undersampled k-space data. The basic working principle originates from the Local All-Pass (LAP) technique, a recently introduced optical flow-based registration. The proposed LAPNet is compared against traditional and deep learning image-based registrations and tested on fully-sampled and highly-accelerated (with two undersampling strategies) 3D respiratory motion-resolved MR images in a cohort of 40 patients with suspected liver or lung metastases and 25 healthy subjects. The proposed LAPNet provided consistent and superior performance to image-based approaches throughout different sampling trajectories and acceleration factors.
Complementary Time-Frequency Domain Networks for Dynamic Parallel MR Image Reconstruction
Dec 22, 2020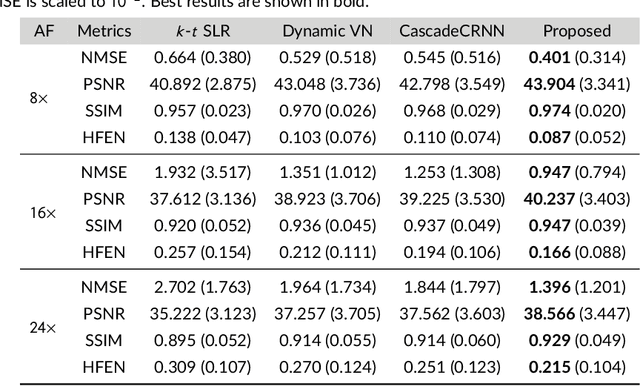
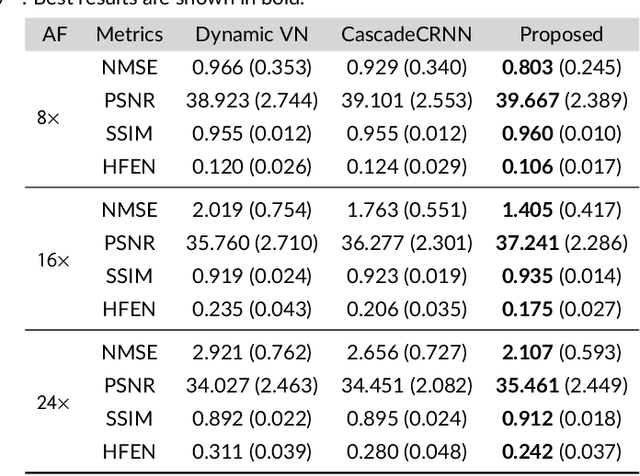
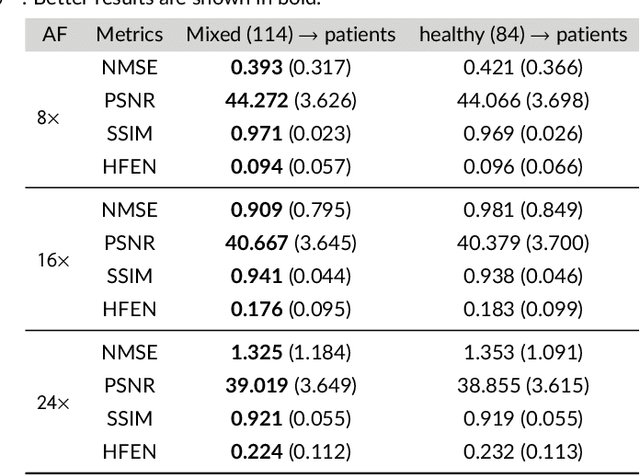
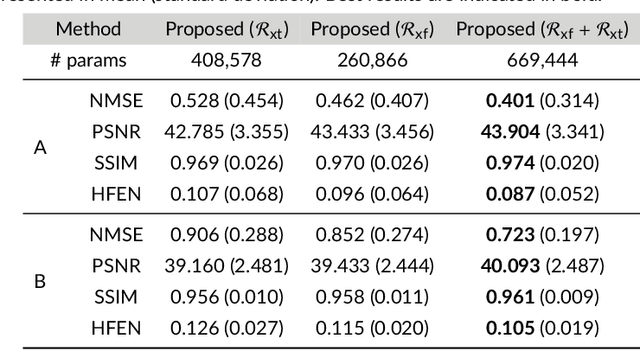
Abstract:Purpose: To introduce a novel deep learning based approach for fast and high-quality dynamic multi-coil MR reconstruction by learning a complementary time-frequency domain network that exploits spatio-temporal correlations simultaneously from complementary domains. Theory and Methods: Dynamic parallel MR image reconstruction is formulated as a multi-variable minimisation problem, where the data is regularised in combined temporal Fourier and spatial (x-f) domain as well as in spatio-temporal image (x-t) domain. An iterative algorithm based on variable splitting technique is derived, which alternates among signal de-aliasing steps in x-f and x-t spaces, a closed-form point-wise data consistency step and a weighted coupling step. The iterative model is embedded into a deep recurrent neural network which learns to recover the image via exploiting spatio-temporal redundancies in complementary domains. Results: Experiments were performed on two datasets of highly undersampled multi-coil short-axis cardiac cine MRI scans. Results demonstrate that our proposed method outperforms the current state-of-the-art approaches both quantitatively and qualitatively. The proposed model can also generalise well to data acquired from a different scanner and data with pathologies that were not seen in the training set. Conclusion: The work shows the benefit of reconstructing dynamic parallel MRI in complementary time-frequency domains with deep neural networks. The method can effectively and robustly reconstruct high-quality images from highly undersampled dynamic multi-coil data ($16 \times$ and $24 \times$ yielding 15s and 10s scan times respectively) with fast reconstruction speed (2.8s). This could potentially facilitate achieving fast single-breath-hold clinical 2D cardiac cine imaging.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge