Zhifan Jiang
FeTTL: Federated Template and Task Learning for Multi-Institutional Medical Imaging
Jan 22, 2026Abstract:Federated learning enables collaborative model training across geographically distributed medical centers while preserving data privacy. However, domain shifts and heterogeneity in data often lead to a degradation in model performance. Medical imaging applications are particularly affected by variations in acquisition protocols, scanner types, and patient populations. To address these issues, we introduce Federated Template and Task Learning (FeTTL), a novel framework designed to harmonize multi-institutional medical imaging data in federated environments. FeTTL learns a global template together with a task model to align data distributions among clients. We evaluated FeTTL on two challenging and diverse multi-institutional medical imaging tasks: retinal fundus optical disc segmentation and histopathological metastasis classification. Experimental results show that FeTTL significantly outperforms the state-of-the-art federated learning baselines (p-values <0.002) for optical disc segmentation and classification of metastases from multi-institutional data. Our experiments further highlight the importance of jointly learning the template and the task. These findings suggest that FeTTL offers a principled and extensible solution for mitigating distribution shifts in federated learning, supporting robust model deployment in real-world, multi-institutional environments.
Adaptable Segmentation Pipeline for Diverse Brain Tumors with Radiomic-guided Subtyping and Lesion-Wise Model Ensemble
Dec 16, 2025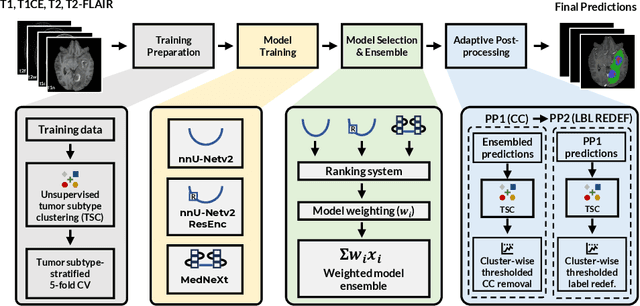
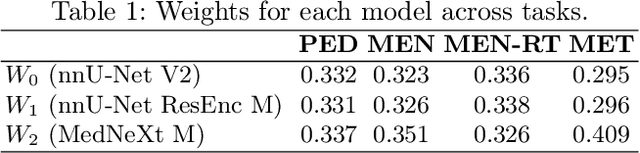
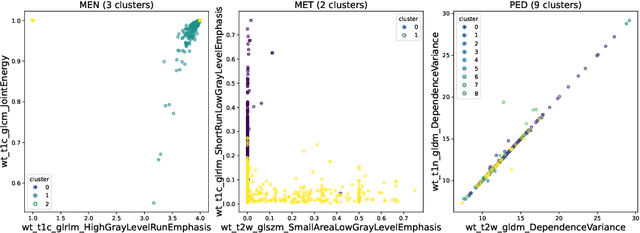
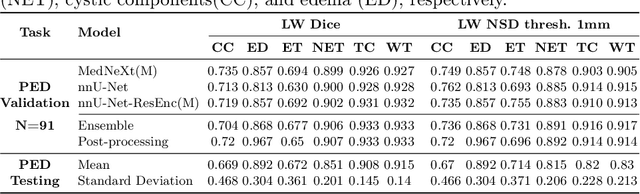
Abstract:Robust and generalizable segmentation of brain tumors on multi-parametric magnetic resonance imaging (MRI) remains difficult because tumor types differ widely. The BraTS 2025 Lighthouse Challenge benchmarks segmentation methods on diverse high-quality datasets of adult and pediatric tumors: multi-consortium international pediatric brain tumor segmentation (PED), preoperative meningioma tumor segmentation (MEN), meningioma radiotherapy segmentation (MEN-RT), and segmentation of pre- and post-treatment brain metastases (MET). We present a flexible, modular, and adaptable pipeline that improves segmentation performance by selecting and combining state-of-the-art models and applying tumor- and lesion-specific processing before and after training. Radiomic features extracted from MRI help detect tumor subtype, ensuring a more balanced training. Custom lesion-level performance metrics determine the influence of each model in the ensemble and optimize post-processing that further refines the predictions, enabling the workflow to tailor every step to each case. On the BraTS testing sets, our pipeline achieved performance comparable to top-ranked algorithms across multiple challenges. These findings confirm that custom lesion-aware processing and model selection yield robust segmentations yet without locking the method to a specific network architecture. Our method has the potential for quantitative tumor measurement in clinical practice, supporting diagnosis and prognosis.
Improving Pre-trained Segmentation Models using Post-Processing
Dec 16, 2025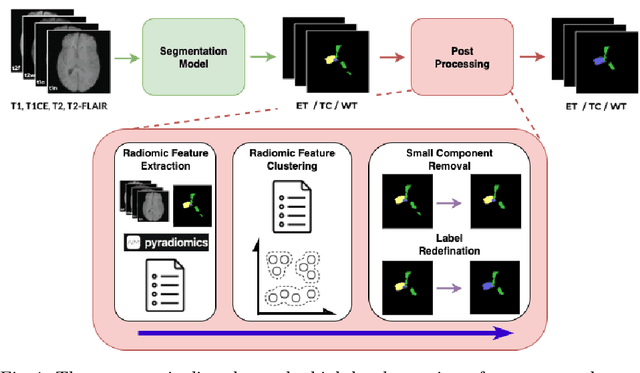
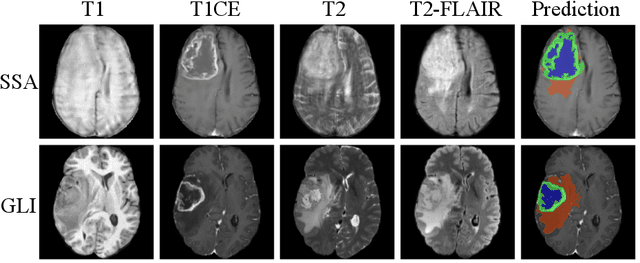

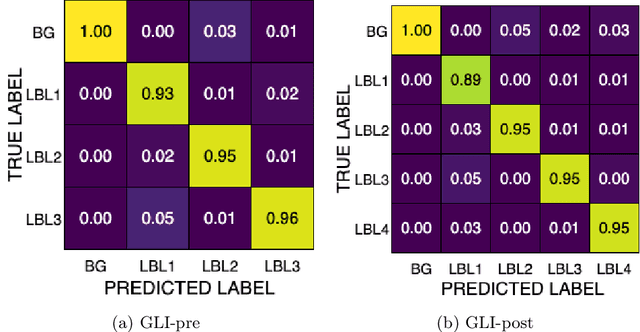
Abstract:Gliomas are the most common malignant brain tumors in adults and are among the most lethal. Despite aggressive treatment, the median survival rate is less than 15 months. Accurate multiparametric MRI (mpMRI) tumor segmentation is critical for surgical planning, radiotherapy, and disease monitoring. While deep learning models have improved the accuracy of automated segmentation, large-scale pre-trained models generalize poorly and often underperform, producing systematic errors such as false positives, label swaps, and slice discontinuities in slices. These limitations are further compounded by unequal access to GPU resources and the growing environmental cost of large-scale model training. In this work, we propose adaptive post-processing techniques to refine the quality of glioma segmentations produced by large-scale pretrained models developed for various types of tumors. We demonstrated the techniques in multiple BraTS 2025 segmentation challenge tasks, with the ranking metric improving by 14.9 % for the sub-Saharan Africa challenge and 0.9% for the adult glioma challenge. This approach promotes a shift in brain tumor segmentation research from increasingly complex model architectures to efficient, clinically aligned post-processing strategies that are precise, computationally fair, and sustainable.
Analysis of the MICCAI Brain Tumor Segmentation -- Metastases (BraTS-METS) 2025 Lighthouse Challenge: Brain Metastasis Segmentation on Pre- and Post-treatment MRI
Apr 16, 2025Abstract:Despite continuous advancements in cancer treatment, brain metastatic disease remains a significant complication of primary cancer and is associated with an unfavorable prognosis. One approach for improving diagnosis, management, and outcomes is to implement algorithms based on artificial intelligence for the automated segmentation of both pre- and post-treatment MRI brain images. Such algorithms rely on volumetric criteria for lesion identification and treatment response assessment, which are still not available in clinical practice. Therefore, it is critical to establish tools for rapid volumetric segmentations methods that can be translated to clinical practice and that are trained on high quality annotated data. The BraTS-METS 2025 Lighthouse Challenge aims to address this critical need by establishing inter-rater and intra-rater variability in dataset annotation by generating high quality annotated datasets from four individual instances of segmentation by neuroradiologists while being recorded on video (two instances doing "from scratch" and two instances after AI pre-segmentation). This high-quality annotated dataset will be used for testing phase in 2025 Lighthouse challenge and will be publicly released at the completion of the challenge. The 2025 Lighthouse challenge will also release the 2023 and 2024 segmented datasets that were annotated using an established pipeline of pre-segmentation, student annotation, two neuroradiologists checking, and one neuroradiologist finalizing the process. It builds upon its previous edition by including post-treatment cases in the dataset. Using these high-quality annotated datasets, the 2025 Lighthouse challenge plans to test benchmark algorithms for automated segmentation of pre-and post-treatment brain metastases (BM), trained on diverse and multi-institutional datasets of MRI images obtained from patients with brain metastases.
Adult Glioma Segmentation in Sub-Saharan Africa using Transfer Learning on Stratified Finetuning Data
Dec 05, 2024



Abstract:Gliomas, a kind of brain tumor characterized by high mortality, present substantial diagnostic challenges in low- and middle-income countries, particularly in Sub-Saharan Africa. This paper introduces a novel approach to glioma segmentation using transfer learning to address challenges in resource-limited regions with minimal and low-quality MRI data. We leverage pre-trained deep learning models, nnU-Net and MedNeXt, and apply a stratified fine-tuning strategy using the BraTS2023-Adult-Glioma and BraTS-Africa datasets. Our method exploits radiomic analysis to create stratified training folds, model training on a large brain tumor dataset, and transfer learning to the Sub-Saharan context. A weighted model ensembling strategy and adaptive post-processing are employed to enhance segmentation accuracy. The evaluation of our proposed method on unseen validation cases on the BraTS-Africa 2024 task resulted in lesion-wise mean Dice scores of 0.870, 0.865, and 0.926, for enhancing tumor, tumor core, and whole tumor regions and was ranked first for the challenge. Our approach highlights the ability of integrated machine-learning techniques to bridge the gap between the medical imaging capabilities of resource-limited countries and established developed regions. By tailoring our methods to a target population's specific needs and constraints, we aim to enhance diagnostic capabilities in isolated environments. Our findings underscore the importance of approaches like local data integration and stratification refinement to address healthcare disparities, ensure practical applicability, and enhance impact.
Magnetic Resonance Imaging Feature-Based Subtyping and Model Ensemble for Enhanced Brain Tumor Segmentation
Dec 05, 2024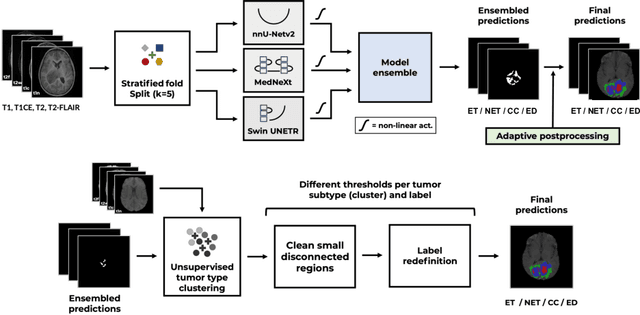
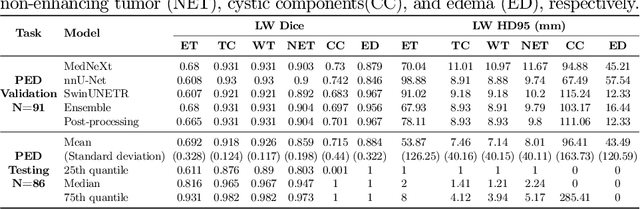
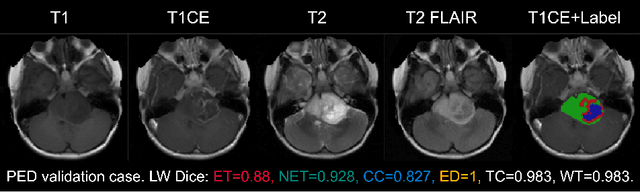
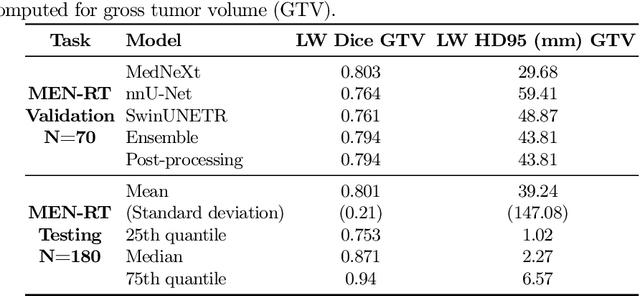
Abstract:Accurate and automatic segmentation of brain tumors in multi-parametric magnetic resonance imaging (mpMRI) is essential for quantitative measurements, which play an increasingly important role in clinical diagnosis and prognosis. The International Brain Tumor Segmentation (BraTS) Challenge 2024 offers a unique benchmarking opportunity, including various types of brain tumors in both adult and pediatric populations, such as pediatric brain tumors (PED), meningiomas (MEN-RT) and brain metastases (MET), among others. Compared to previous editions, BraTS 2024 has implemented changes to substantially increase clinical relevance, such as refined tumor regions for evaluation. We propose a deep learning-based ensemble approach that integrates state-of-the-art segmentation models. Additionally, we introduce innovative, adaptive pre- and post-processing techniques that employ MRI-based radiomic analyses to differentiate tumor subtypes. Given the heterogeneous nature of the tumors present in the BraTS datasets, this approach enhances the precision and generalizability of segmentation models. On the final testing sets, our method achieved mean lesion-wise Dice similarity coefficients of 0.926, 0.801, and 0.688 for the whole tumor in PED, MEN-RT, and MET, respectively. These results demonstrate the effectiveness of our approach in improving segmentation performance and generalizability for various brain tumor types.
Model Ensemble for Brain Tumor Segmentation in Magnetic Resonance Imaging
Sep 12, 2024



Abstract:Segmenting brain tumors in multi-parametric magnetic resonance imaging enables performing quantitative analysis in support of clinical trials and personalized patient care. This analysis provides the potential to impact clinical decision-making processes, including diagnosis and prognosis. In 2023, the well-established Brain Tumor Segmentation (BraTS) challenge presented a substantial expansion with eight tasks and 4,500 brain tumor cases. In this paper, we present a deep learning-based ensemble strategy that is evaluated for newly included tumor cases in three tasks: pediatric brain tumors (PED), intracranial meningioma (MEN), and brain metastases (MET). In particular, we ensemble outputs from state-of-the-art nnU-Net and Swin UNETR models on a region-wise basis. Furthermore, we implemented a targeted post-processing strategy based on a cross-validated threshold search to improve the segmentation results for tumor sub-regions. The evaluation of our proposed method on unseen test cases for the three tasks resulted in lesion-wise Dice scores for PED: 0.653, 0.809, 0.826; MEN: 0.876, 0.867, 0.849; and MET: 0.555, 0.6, 0.58; for the enhancing tumor, tumor core, and whole tumor, respectively. Our method was ranked first for PED, third for MEN, and fourth for MET, respectively.
Unifying Invariant and Variant Features for Graph Out-of-Distribution via Probability of Necessity and Sufficiency
Jul 21, 2024



Abstract:Graph Out-of-Distribution (OOD), requiring that models trained on biased data generalize to the unseen test data, has considerable real-world applications. One of the most mainstream methods is to extract the invariant subgraph by aligning the original and augmented data with the help of environment augmentation. However, these solutions might lead to the loss or redundancy of semantic subgraphs and result in suboptimal generalization. To address this challenge, we propose exploiting Probability of Necessity and Sufficiency (PNS) to extract sufficient and necessary invariant substructures. Beyond that, we further leverage the domain variant subgraphs related to the labels to boost the generalization performance in an ensemble manner. Specifically, we first consider the data generation process for graph data. Under mild conditions, we show that the sufficient and necessary invariant subgraph can be extracted by minimizing an upper bound, built on the theoretical advance of the probability of necessity and sufficiency. To further bridge the theory and algorithm, we devise the model called Sufficiency and Necessity Inspired Graph Learning (SNIGL), which ensembles an invariant subgraph classifier on top of latent sufficient and necessary invariant subgraphs, and a domain variant subgraph classifier specific to the test domain for generalization enhancement. Experimental results demonstrate that our SNIGL model outperforms the state-of-the-art techniques on six public benchmarks, highlighting its effectiveness in real-world scenarios.
Data Alchemy: Mitigating Cross-Site Model Variability Through Test Time Data Calibration
Jul 18, 2024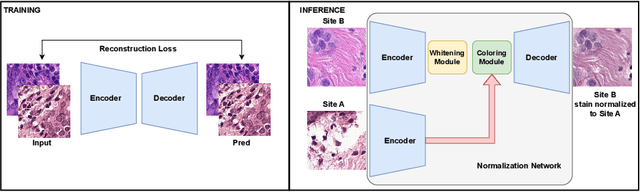
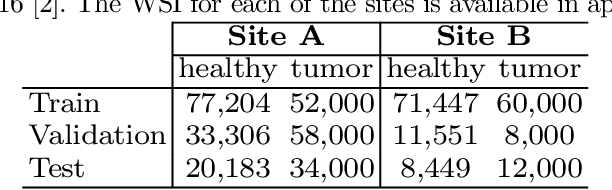
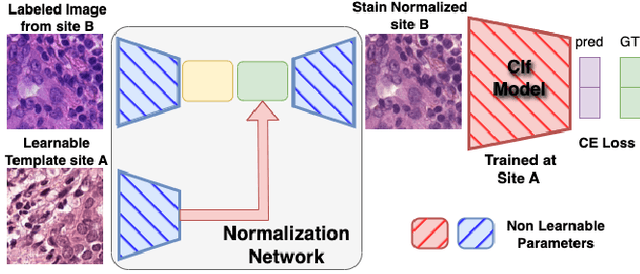
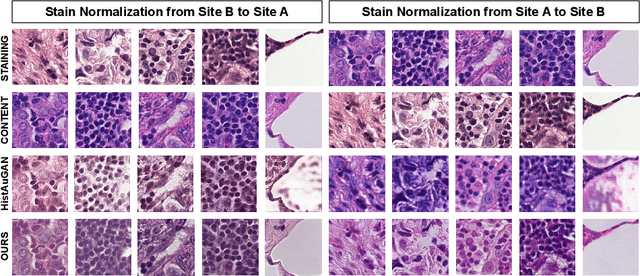
Abstract:Deploying deep learning-based imaging tools across various clinical sites poses significant challenges due to inherent domain shifts and regulatory hurdles associated with site-specific fine-tuning. For histopathology, stain normalization techniques can mitigate discrepancies, but they often fall short of eliminating inter-site variations. Therefore, we present Data Alchemy, an explainable stain normalization method combined with test time data calibration via a template learning framework to overcome barriers in cross-site analysis. Data Alchemy handles shifts inherent to multi-site data and minimizes them without needing to change the weights of the normalization or classifier networks. Our approach extends to unseen sites in various clinical settings where data domain discrepancies are unknown. Extensive experiments highlight the efficacy of our framework in tumor classification in hematoxylin and eosin-stained patches. Our explainable normalization method boosts classification tasks' area under the precision-recall curve(AUPR) by 0.165, 0.545 to 0.710. Additionally, Data Alchemy further reduces the multisite classification domain gap, by improving the 0.710 AUPR an additional 0.142, elevating classification performance further to 0.852, from 0.545. Our Data Alchemy framework can popularize precision medicine with minimal operational overhead by allowing for the seamless integration of pre-trained deep learning-based clinical tools across multiple sites.
BraTS-PEDs: Results of the Multi-Consortium International Pediatric Brain Tumor Segmentation Challenge 2023
Jul 11, 2024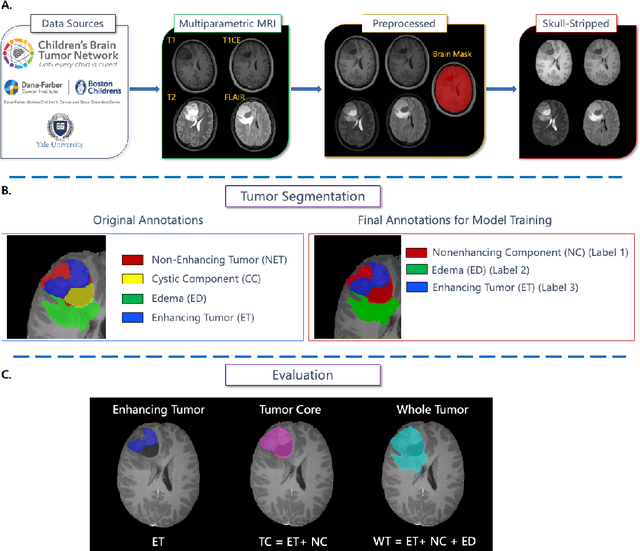

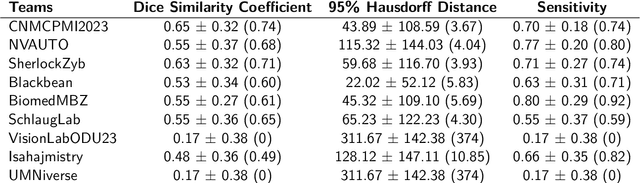
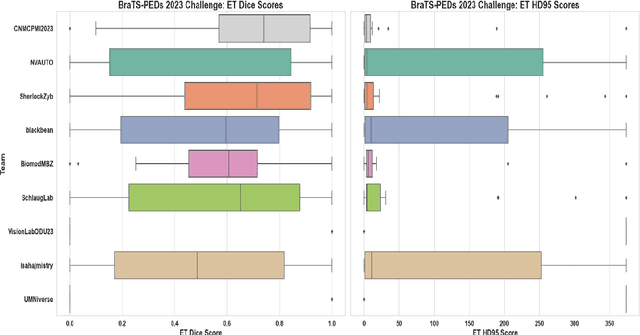
Abstract:Pediatric central nervous system tumors are the leading cause of cancer-related deaths in children. The five-year survival rate for high-grade glioma in children is less than 20%. The development of new treatments is dependent upon multi-institutional collaborative clinical trials requiring reproducible and accurate centralized response assessment. We present the results of the BraTS-PEDs 2023 challenge, the first Brain Tumor Segmentation (BraTS) challenge focused on pediatric brain tumors. This challenge utilized data acquired from multiple international consortia dedicated to pediatric neuro-oncology and clinical trials. BraTS-PEDs 2023 aimed to evaluate volumetric segmentation algorithms for pediatric brain gliomas from magnetic resonance imaging using standardized quantitative performance evaluation metrics employed across the BraTS 2023 challenges. The top-performing AI approaches for pediatric tumor analysis included ensembles of nnU-Net and Swin UNETR, Auto3DSeg, or nnU-Net with a self-supervised framework. The BraTSPEDs 2023 challenge fostered collaboration between clinicians (neuro-oncologists, neuroradiologists) and AI/imaging scientists, promoting faster data sharing and the development of automated volumetric analysis techniques. These advancements could significantly benefit clinical trials and improve the care of children with brain tumors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge