Anna Zapaishchykova
Artificial Intelligence in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, MA, USA, Department of Radiation Oncology, Dana-Farber Cancer Institute and Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA, Radiology and Nuclear Medicine, CARIM & GROW, Maastricht University, Maastricht, the Netherlands
TissUnet: Improved Extracranial Tissue and Cranium Segmentation for Children through Adulthood
Jun 06, 2025



Abstract:Extracranial tissues visible on brain magnetic resonance imaging (MRI) may hold significant value for characterizing health conditions and clinical decision-making, yet they are rarely quantified. Current tools have not been widely validated, particularly in settings of developing brains or underlying pathology. We present TissUnet, a deep learning model that segments skull bone, subcutaneous fat, and muscle from routine three-dimensional T1-weighted MRI, with or without contrast enhancement. The model was trained on 155 paired MRI-computed tomography (CT) scans and validated across nine datasets covering a wide age range and including individuals with brain tumors. In comparison to AI-CT-derived labels from 37 MRI-CT pairs, TissUnet achieved a median Dice coefficient of 0.79 [IQR: 0.77-0.81] in a healthy adult cohort. In a second validation using expert manual annotations, median Dice was 0.83 [IQR: 0.83-0.84] in healthy individuals and 0.81 [IQR: 0.78-0.83] in tumor cases, outperforming previous state-of-the-art method. Acceptability testing resulted in an 89% acceptance rate after adjudication by a tie-breaker(N=108 MRIs), and TissUnet demonstrated excellent performance in the blinded comparative review (N=45 MRIs), including both healthy and tumor cases in pediatric populations. TissUnet enables fast, accurate, and reproducible segmentation of extracranial tissues, supporting large-scale studies on craniofacial morphology, treatment effects, and cardiometabolic risk using standard brain T1w MRI.
BraTS-PEDs: Results of the Multi-Consortium International Pediatric Brain Tumor Segmentation Challenge 2023
Jul 11, 2024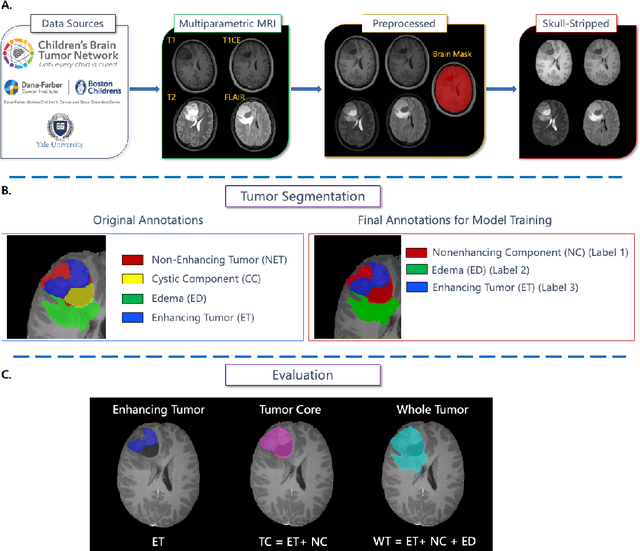

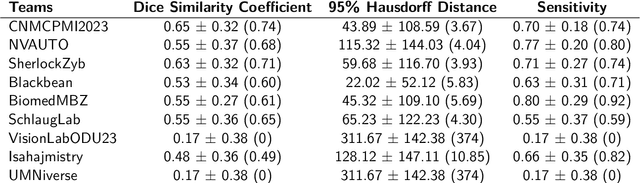
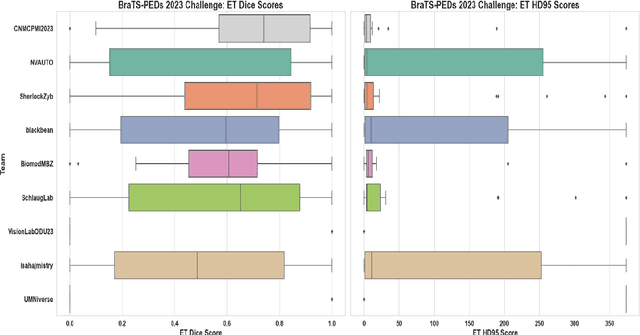
Abstract:Pediatric central nervous system tumors are the leading cause of cancer-related deaths in children. The five-year survival rate for high-grade glioma in children is less than 20%. The development of new treatments is dependent upon multi-institutional collaborative clinical trials requiring reproducible and accurate centralized response assessment. We present the results of the BraTS-PEDs 2023 challenge, the first Brain Tumor Segmentation (BraTS) challenge focused on pediatric brain tumors. This challenge utilized data acquired from multiple international consortia dedicated to pediatric neuro-oncology and clinical trials. BraTS-PEDs 2023 aimed to evaluate volumetric segmentation algorithms for pediatric brain gliomas from magnetic resonance imaging using standardized quantitative performance evaluation metrics employed across the BraTS 2023 challenges. The top-performing AI approaches for pediatric tumor analysis included ensembles of nnU-Net and Swin UNETR, Auto3DSeg, or nnU-Net with a self-supervised framework. The BraTSPEDs 2023 challenge fostered collaboration between clinicians (neuro-oncologists, neuroradiologists) and AI/imaging scientists, promoting faster data sharing and the development of automated volumetric analysis techniques. These advancements could significantly benefit clinical trials and improve the care of children with brain tumors.
The Brain Tumor Segmentation in Pediatrics (BraTS-PEDs) Challenge: Focus on Pediatrics (CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs)
Apr 29, 2024Abstract:Pediatric tumors of the central nervous system are the most common cause of cancer-related death in children. The five-year survival rate for high-grade gliomas in children is less than 20%. Due to their rarity, the diagnosis of these entities is often delayed, their treatment is mainly based on historic treatment concepts, and clinical trials require multi-institutional collaborations. Here we present the CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs challenge, focused on pediatric brain tumors with data acquired across multiple international consortia dedicated to pediatric neuro-oncology and clinical trials. The CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs challenge brings together clinicians and AI/imaging scientists to lead to faster development of automated segmentation techniques that could benefit clinical trials, and ultimately the care of children with brain tumors.
Magnetic resonance delta radiomics to track radiation response in lung tumors receiving stereotactic MRI-guided radiotherapy
Feb 23, 2024



Abstract:Introduction: Lung cancer is a leading cause of cancer-related mortality, and stereotactic body radiotherapy (SBRT) has become a standard treatment for early-stage lung cancer. However, the heterogeneous response to radiation at the tumor level poses challenges. Currently, standardized dosage regimens lack adaptation based on individual patient or tumor characteristics. Thus, we explore the potential of delta radiomics from on-treatment magnetic resonance (MR) imaging to track radiation dose response, inform personalized radiotherapy dosing, and predict outcomes. Methods: A retrospective study of 47 MR-guided lung SBRT treatments for 39 patients was conducted. Radiomic features were extracted using Pyradiomics, and stability was evaluated temporally and spatially. Delta radiomics were correlated with radiation dose delivery and assessed for associations with tumor control and survival with Cox regressions. Results: Among 107 features, 49 demonstrated temporal stability, and 57 showed spatial stability. Fifteen stable and non-collinear features were analyzed. Median Skewness and surface to volume ratio decreased with radiation dose fraction delivery, while coarseness and 90th percentile values increased. Skewness had the largest relative median absolute changes (22%-45%) per fraction from baseline and was associated with locoregional failure (p=0.012) by analysis of covariance. Skewness, Elongation, and Flatness were significantly associated with local recurrence-free survival, while tumor diameter and volume were not. Conclusions: Our study establishes the feasibility and stability of delta radiomics analysis for MR-guided lung SBRT. Findings suggest that MR delta radiomics can capture short-term radiographic manifestations of intra-tumoral radiation effect.
The Brain Tumor Segmentation Challenge 2023: Focus on Pediatrics
May 26, 2023Abstract:Pediatric tumors of the central nervous system are the most common cause of cancer-related death in children. The five-year survival rate for high-grade gliomas in children is less than 20\%. Due to their rarity, the diagnosis of these entities is often delayed, their treatment is mainly based on historic treatment concepts, and clinical trials require multi-institutional collaborations. The MICCAI Brain Tumor Segmentation (BraTS) Challenge is a landmark community benchmark event with a successful history of 12 years of resource creation for the segmentation and analysis of adult glioma. Here we present the CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs 2023 challenge, which represents the first BraTS challenge focused on pediatric brain tumors with data acquired across multiple international consortia dedicated to pediatric neuro-oncology and clinical trials. The BraTS-PEDs 2023 challenge focuses on benchmarking the development of volumentric segmentation algorithms for pediatric brain glioma through standardized quantitative performance evaluation metrics utilized across the BraTS 2023 cluster of challenges. Models gaining knowledge from the BraTS-PEDs multi-parametric structural MRI (mpMRI) training data will be evaluated on separate validation and unseen test mpMRI dataof high-grade pediatric glioma. The CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs 2023 challenge brings together clinicians and AI/imaging scientists to lead to faster development of automated segmentation techniques that could benefit clinical trials, and ultimately the care of children with brain tumors.
The Ability of Image-Language Explainable Models to Resemble Domain Expertise
Sep 19, 2022



Abstract:Recent advances in vision and language (V+L) models have a promising impact in the healthcare field. However, such models struggle to explain how and why a particular decision was made. In addition, model transparency and involvement of domain expertise are critical success factors for machine learning models to make an entrance into the field. In this work, we study the use of the local surrogate explainability technique to overcome the problem of black-box deep learning models. We explore the feasibility of resembling domain expertise using the local surrogates in combination with an underlying V+L to generate multi-modal visual and language explanations. We demonstrate that such explanations can serve as helpful feedback in guiding model training for data scientists and machine learning engineers in the field.
An Interpretable Approach to Automated Severity Scoring in Pelvic Trauma
May 21, 2021



Abstract:Pelvic ring disruptions result from blunt injury mechanisms and are often found in patients with multi-system trauma. To grade pelvic fracture severity in trauma victims based on whole-body CT, the Tile AO/OTA classification is frequently used. Due to the high volume of whole-body trauma CTs generated in busy trauma centers, an automated approach to Tile classification would provide substantial value, e.,g., to prioritize the reading queue of the attending trauma radiologist. In such scenario, an automated method should perform grading based on a transparent process and based on interpretable features to enable interaction with human readers and lower their workload by offering insights from a first automated read of the scan. This paper introduces an automated yet interpretable pelvic trauma decision support system to assist radiologists in fracture detection and Tile grade classification. The method operates similarly to human interpretation of CT scans and first detects distinct pelvic fractures on CT with high specificity using a Faster-RCNN model that are then interpreted using a structural causal model based on clinical best practices to infer an initial Tile grade. The Bayesian causal model and finally, the object detector are then queried for likely co-occurring fractures that may have been rejected initially due to the highly specific operating point of the detector, resulting in an updated list of detected fractures and corresponding final Tile grade. Our method is transparent in that it provides finding location and type using the object detector, as well as information on important counterfactuals that would invalidate the system's recommendation and achieves an AUC of 83.3%/85.1% for translational/rotational instability. Despite being designed for human-machine teaming, our approach does not compromise on performance compared to previous black-box approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge