Sachin Jambawalikar
Cyst-X: AI-Powered Pancreatic Cancer Risk Prediction from Multicenter MRI in Centralized and Federated Learning
Jul 29, 2025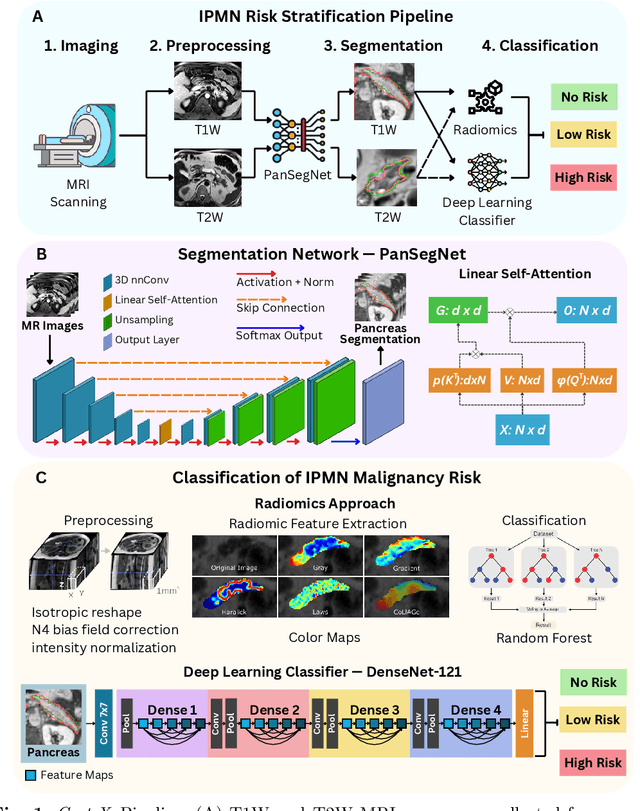

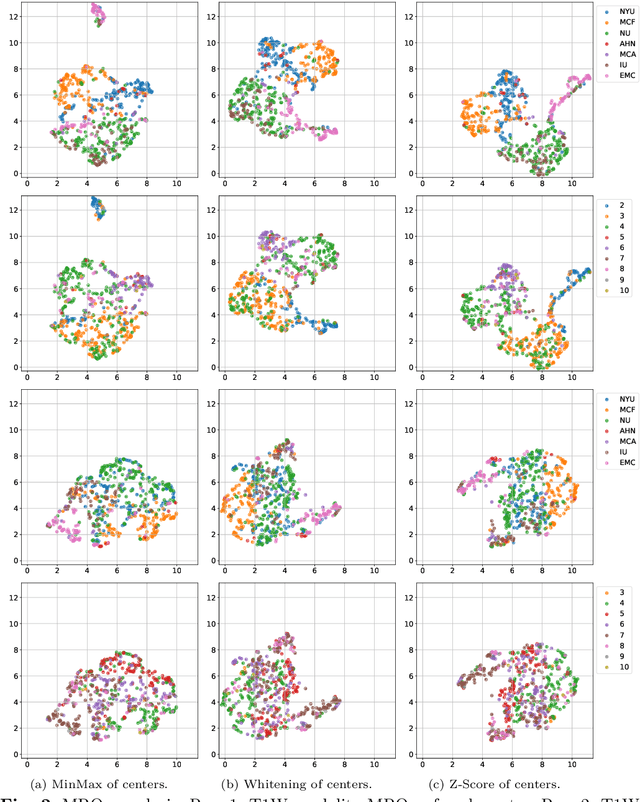
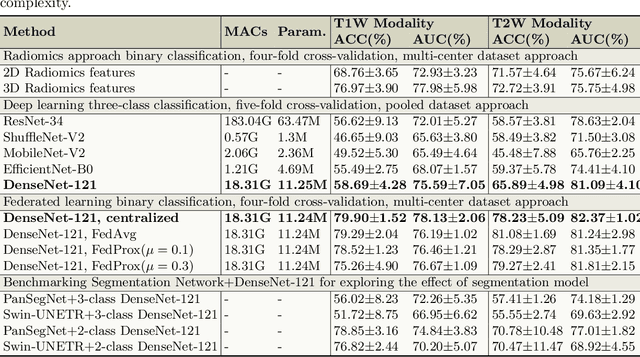
Abstract:Pancreatic cancer is projected to become the second-deadliest malignancy in Western countries by 2030, highlighting the urgent need for better early detection. Intraductal papillary mucinous neoplasms (IPMNs), key precursors to pancreatic cancer, are challenging to assess with current guidelines, often leading to unnecessary surgeries or missed malignancies. We present Cyst-X, an AI framework that predicts IPMN malignancy using multicenter MRI data, leveraging MRI's superior soft tissue contrast over CT. Trained on 723 T1- and 738 T2-weighted scans from 764 patients across seven institutions, our models (AUC=0.82) significantly outperform both Kyoto guidelines (AUC=0.75) and expert radiologists. The AI-derived imaging features align with known clinical markers and offer biologically meaningful insights. We also demonstrate strong performance in a federated learning setting, enabling collaborative training without sharing patient data. To promote privacy-preserving AI development and improve IPMN risk stratification, the Cyst-X dataset is released as the first large-scale, multi-center pancreatic cysts MRI dataset.
Predicting Risk of Pulmonary Fibrosis Formation in PASC Patients
May 15, 2025Abstract:While the acute phase of the COVID-19 pandemic has subsided, its long-term effects persist through Post-Acute Sequelae of COVID-19 (PASC), commonly known as Long COVID. There remains substantial uncertainty regarding both its duration and optimal management strategies. PASC manifests as a diverse array of persistent or newly emerging symptoms--ranging from fatigue, dyspnea, and neurologic impairments (e.g., brain fog), to cardiovascular, pulmonary, and musculoskeletal abnormalities--that extend beyond the acute infection phase. This heterogeneous presentation poses substantial challenges for clinical assessment, diagnosis, and treatment planning. In this paper, we focus on imaging findings that may suggest fibrotic damage in the lungs, a critical manifestation characterized by scarring of lung tissue, which can potentially affect long-term respiratory function in patients with PASC. This study introduces a novel multi-center chest CT analysis framework that combines deep learning and radiomics for fibrosis prediction. Our approach leverages convolutional neural networks (CNNs) and interpretable feature extraction, achieving 82.2% accuracy and 85.5% AUC in classification tasks. We demonstrate the effectiveness of Grad-CAM visualization and radiomics-based feature analysis in providing clinically relevant insights for PASC-related lung fibrosis prediction. Our findings highlight the potential of deep learning-driven computational methods for early detection and risk assessment of PASC-related lung fibrosis--presented for the first time in the literature.
Information Bottleneck Attribution for Visual Explanations of Diagnosis and Prognosis
Apr 07, 2021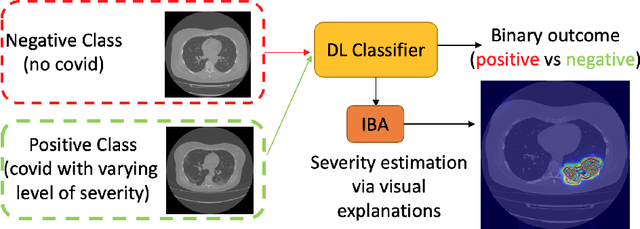



Abstract:Visual explanation methods have an important role in the prognosis of the patients where the annotated data is limited or not available. There have been several attempts to use gradient-based attribution methods to localize pathology from medical scans without using segmentation labels. This research direction has been impeded by the lack of robustness and reliability. These methods are highly sensitive to the network parameters. In this study, we introduce a robust visual explanation method to address this problem for medical applications. We provide a highly innovative algorithm to quantifying lesions in the lungs caused by the Covid-19 with high accuracy and robustness without using dense segmentation labels. Inspired by the information bottleneck concept, we mask the neural network representation with noise to find out important regions. This approach overcomes the drawbacks of commonly used Grad-Cam and its derived algorithms. The premise behind our proposed strategy is that the information flow is minimized while ensuring the classifier prediction stays similar. Our findings indicate that the bottleneck condition provides a more stable and robust severity estimation than the similar attribution methods.
The International Workshop on Osteoarthritis Imaging Knee MRI Segmentation Challenge: A Multi-Institute Evaluation and Analysis Framework on a Standardized Dataset
May 26, 2020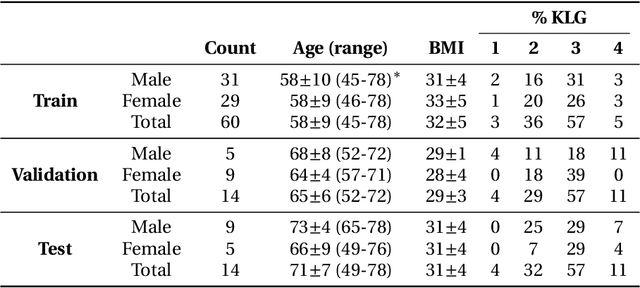
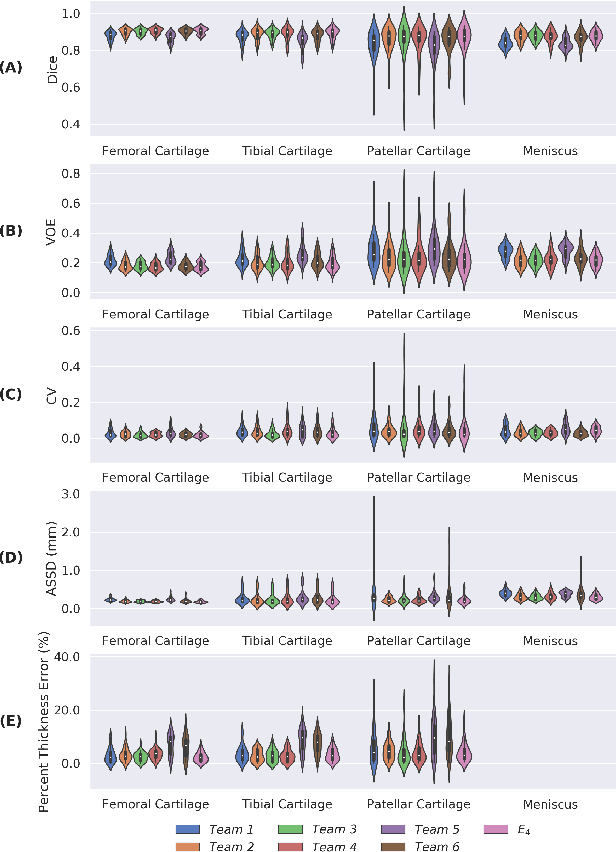
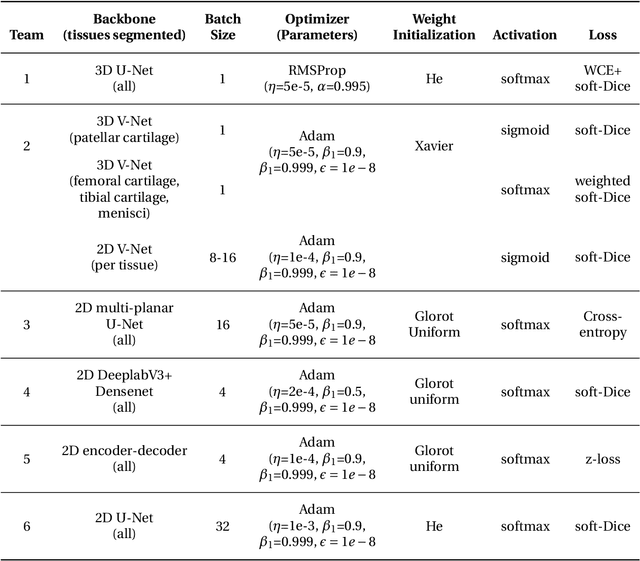
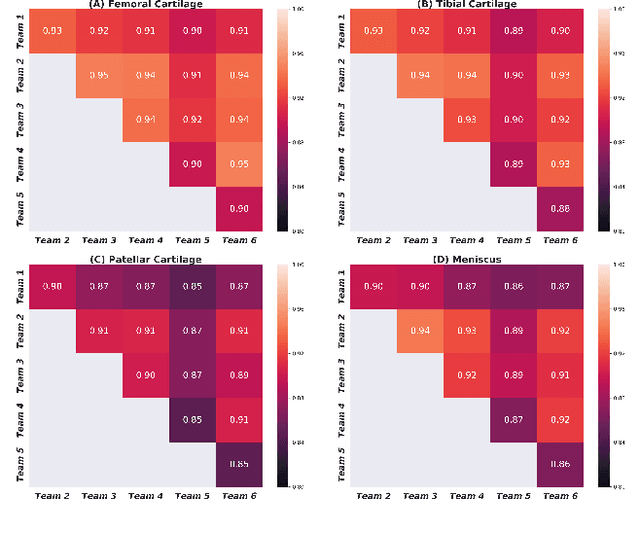
Abstract:Purpose: To organize a knee MRI segmentation challenge for characterizing the semantic and clinical efficacy of automatic segmentation methods relevant for monitoring osteoarthritis progression. Methods: A dataset partition consisting of 3D knee MRI from 88 subjects at two timepoints with ground-truth articular (femoral, tibial, patellar) cartilage and meniscus segmentations was standardized. Challenge submissions and a majority-vote ensemble were evaluated using Dice score, average symmetric surface distance, volumetric overlap error, and coefficient of variation on a hold-out test set. Similarities in network segmentations were evaluated using pairwise Dice correlations. Articular cartilage thickness was computed per-scan and longitudinally. Correlation between thickness error and segmentation metrics was measured using Pearson's coefficient. Two empirical upper bounds for ensemble performance were computed using combinations of model outputs that consolidated true positives and true negatives. Results: Six teams (T1-T6) submitted entries for the challenge. No significant differences were observed across all segmentation metrics for all tissues (p=1.0) among the four top-performing networks (T2, T3, T4, T6). Dice correlations between network pairs were high (>0.85). Per-scan thickness errors were negligible among T1-T4 (p=0.99) and longitudinal changes showed minimal bias (<0.03mm). Low correlations (<0.41) were observed between segmentation metrics and thickness error. The majority-vote ensemble was comparable to top performing networks (p=1.0). Empirical upper bound performances were similar for both combinations (p=1.0). Conclusion: Diverse networks learned to segment the knee similarly where high segmentation accuracy did not correlate to cartilage thickness accuracy. Voting ensembles did not outperform individual networks but may help regularize individual models.
Cross-modality Knowledge Transfer for Prostate Segmentation from CT Scans
Sep 11, 2019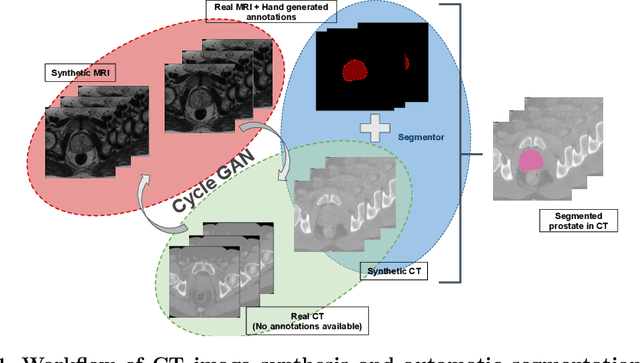


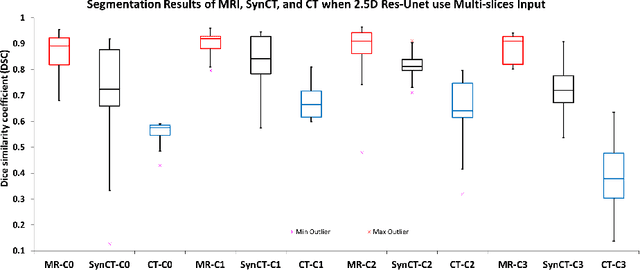
Abstract:Creating large scale high-quality annotations is a known challenge in medical imaging. In this work, based on the CycleGAN algorithm, we propose leveraging annotations from one modality to be useful in other modalities. More specifically, the proposed algorithm creates highly realistic synthetic CT images (SynCT) from prostate MR images using unpaired data sets. By using SynCT images (without segmentation labels) and MR images (with segmentation labels available), we have trained a deep segmentation network for precise delineation of prostate from real CT scans. For the generator in our CycleGAN, the cycle consistency term is used to guarantee that SynCT shares the identical manually-drawn, high-quality masks originally delineated on MR images. Further, we introduce a cost function based on structural similarity index (SSIM) to improve the anatomical similarity between real and synthetic images. For segmentation followed by the SynCT generation from CycleGAN, automatic delineation is achieved through a 2.5D Residual U-Net. Quantitative evaluation demonstrates comparable segmentation results between our SynCT and radiologist drawn masks for real CT images, solving an important problem in medical image segmentation field when ground truth annotations are not available for the modality of interest.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge