Ruoxuan Xiong
Sequential Treatment Effect Estimation with Unmeasured Confounders
May 14, 2025Abstract:This paper studies the cumulative causal effects of sequential treatments in the presence of unmeasured confounders. It is a critical issue in sequential decision-making scenarios where treatment decisions and outcomes dynamically evolve over time. Advanced causal methods apply transformer as a backbone to model such time sequences, which shows superiority in capturing long time dependence and periodic patterns via attention mechanism. However, even they control the observed confounding, these estimators still suffer from unmeasured confounders, which influence both treatment assignments and outcomes. How to adjust the latent confounding bias in sequential treatment effect estimation remains an open challenge. Therefore, we propose a novel Decomposing Sequential Instrumental Variable framework for CounterFactual Regression (DSIV-CFR), relying on a common negative control assumption. Specifically, an instrumental variable (IV) is a special negative control exposure, while the previous outcome serves as a negative control outcome. This allows us to recover the IVs latent in observation variables and estimate sequential treatment effects via a generalized moment condition. We conducted experiments on 4 datasets and achieved significant performance in one- and multi-step prediction, supported by which we can identify optimal treatments for dynamic systems.
Can We Validate Counterfactual Estimations in the Presence of General Network Interference?
Feb 03, 2025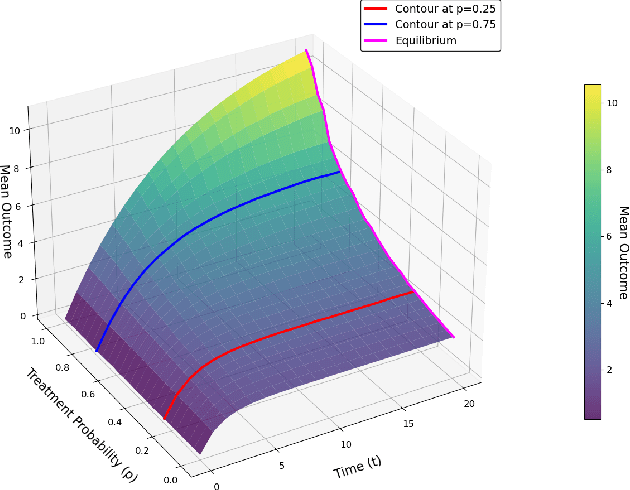
Abstract:In experimental settings with network interference, a unit's treatment can influence outcomes of other units, challenging both causal effect estimation and its validation. Classic validation approaches fail as outcomes are only observable under one treatment scenario and exhibit complex correlation patterns due to interference. To address these challenges, we introduce a new framework enabling cross-validation for counterfactual estimation. At its core is our distribution-preserving network bootstrap method -- a theoretically-grounded approach inspired by approximate message passing. This method creates multiple subpopulations while preserving the underlying distribution of network effects. We extend recent causal message-passing developments by incorporating heterogeneous unit-level characteristics and varying local interactions, ensuring reliable finite-sample performance through non-asymptotic analysis. We also develop and publicly release a comprehensive benchmark toolbox with diverse experimental environments, from networks of interacting AI agents to opinion formation in real-world communities and ride-sharing applications. These environments provide known ground truth values while maintaining realistic complexities, enabling systematic examination of causal inference methods. Extensive evaluation across these environments demonstrates our method's robustness to diverse forms of network interference. Our work provides researchers with both a practical estimation framework and a standardized platform for testing future methodological developments.
Higher-Order Causal Message Passing for Experimentation with Complex Interference
Nov 01, 2024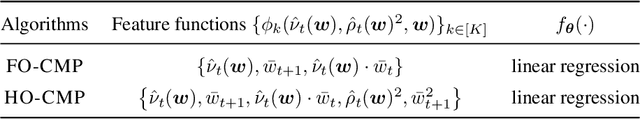
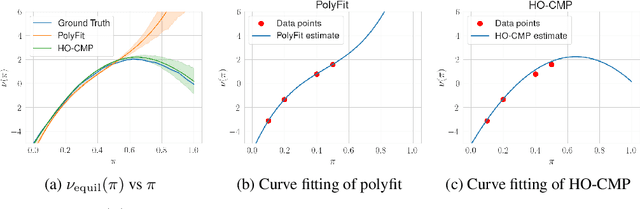
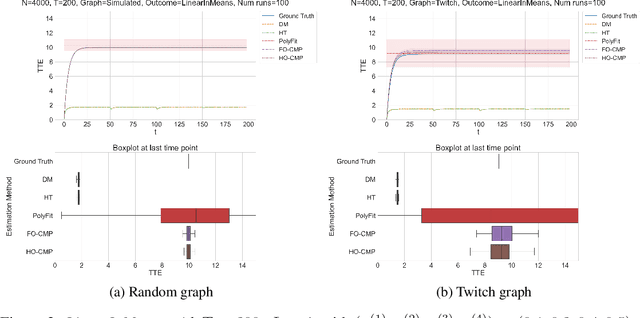
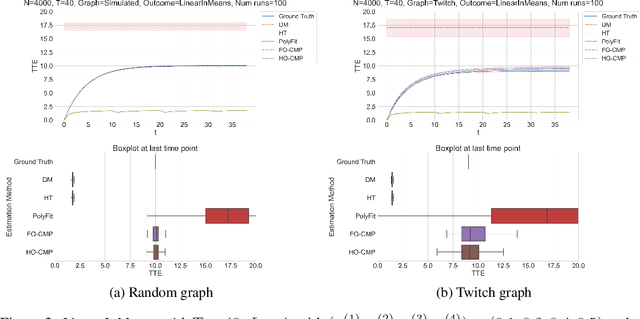
Abstract:Accurate estimation of treatment effects is essential for decision-making across various scientific fields. This task, however, becomes challenging in areas like social sciences and online marketplaces, where treating one experimental unit can influence outcomes for others through direct or indirect interactions. Such interference can lead to biased treatment effect estimates, particularly when the structure of these interactions is unknown. We address this challenge by introducing a new class of estimators based on causal message-passing, specifically designed for settings with pervasive, unknown interference. Our estimator draws on information from the sample mean and variance of unit outcomes and treatments over time, enabling efficient use of observed data to estimate the evolution of the system state. Concretely, we construct non-linear features from the moments of unit outcomes and treatments and then learn a function that maps these features to future mean and variance of unit outcomes. This allows for the estimation of the treatment effect over time. Extensive simulations across multiple domains, using synthetic and real network data, demonstrate the efficacy of our approach in estimating total treatment effect dynamics, even in cases where interference exhibits non-monotonic behavior in the probability of treatment.
Generalized Encouragement-Based Instrumental Variables for Counterfactual Regression
Aug 10, 2024



Abstract:In causal inference, encouragement designs (EDs) are widely used to analyze causal effects, when randomized controlled trials (RCTs) are impractical or compliance to treatment cannot be perfectly enforced. Unlike RCTs, which directly allocate treatments, EDs randomly assign encouragement policies that positively motivate individuals to engage in a specific treatment. These random encouragements act as instrumental variables (IVs), facilitating the identification of causal effects through leveraging exogenous perturbations in discrete treatment scenarios. However, real-world applications of encouragement designs often face challenges such as incomplete randomization, limited experimental data, and significantly fewer encouragements compared to treatments, hindering precise causal effect estimation. To address this, this paper introduces novel theories and algorithms for identifying the Conditional Average Treatment Effect (CATE) using variations in encouragement. Further, by leveraging both observational and encouragement data, we propose a generalized IV estimator, named Encouragement-based Counterfactual Regression (EnCounteR), to effectively estimate the causal effects. Extensive experiments on both synthetic and real-world datasets demonstrate the superiority of EnCounteR over existing methods.
Causal Inference with Complex Treatments: A Survey
Jul 19, 2024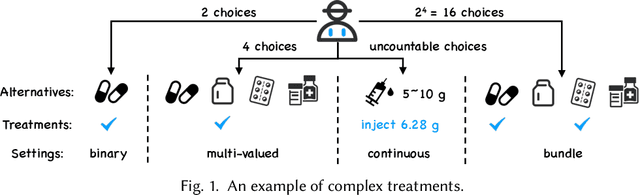
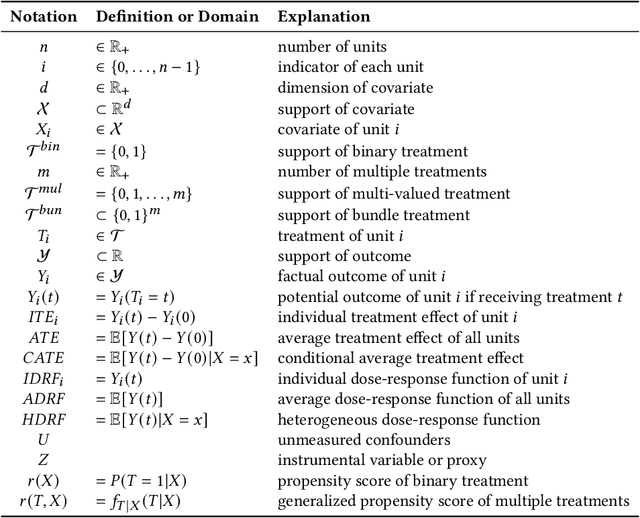
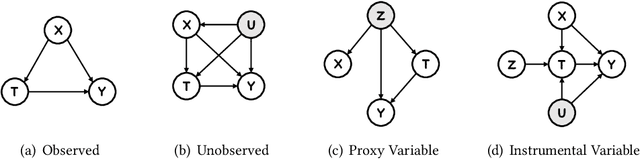
Abstract:Causal inference plays an important role in explanatory analysis and decision making across various fields like statistics, marketing, health care, and education. Its main task is to estimate treatment effects and make intervention policies. Traditionally, most of the previous works typically focus on the binary treatment setting that there is only one treatment for a unit to adopt or not. However, in practice, the treatment can be much more complex, encompassing multi-valued, continuous, or bundle options. In this paper, we refer to these as complex treatments and systematically and comprehensively review the causal inference methods for addressing them. First, we formally revisit the problem definition, the basic assumptions, and their possible variations under specific conditions. Second, we sequentially review the related methods for multi-valued, continuous, and bundled treatment settings. In each situation, we tentatively divide the methods into two categories: those conforming to the unconfoundedness assumption and those violating it. Subsequently, we discuss the available datasets and open-source codes. Finally, we provide a brief summary of these works and suggest potential directions for future research.
Data-Driven Switchback Experiments: Theoretical Tradeoffs and Empirical Bayes Designs
Jun 10, 2024Abstract:We study the design and analysis of switchback experiments conducted on a single aggregate unit. The design problem is to partition the continuous time space into intervals and switch treatments between intervals, in order to minimize the estimation error of the treatment effect. We show that the estimation error depends on four factors: carryover effects, periodicity, serially correlated outcomes, and impacts from simultaneous experiments. We derive a rigorous bias-variance decomposition and show the tradeoffs of the estimation error from these factors. The decomposition provides three new insights in choosing a design: First, balancing the periodicity between treated and control intervals reduces the variance; second, switching less frequently reduces the bias from carryover effects while increasing the variance from correlated outcomes, and vice versa; third, randomizing interval start and end points reduces both bias and variance from simultaneous experiments. Combining these insights, we propose a new empirical Bayes design approach. This approach uses prior data and experiments for designing future experiments. We illustrate this approach using real data from a ride-sharing platform, yielding a design that reduces MSE by 33% compared to the status quo design used on the platform.
Contrastive Balancing Representation Learning for Heterogeneous Dose-Response Curves Estimation
Mar 21, 2024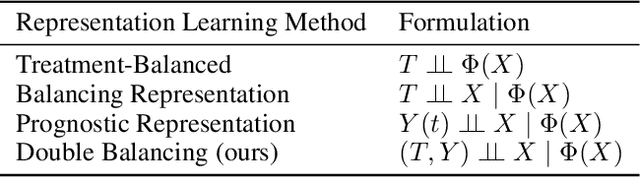
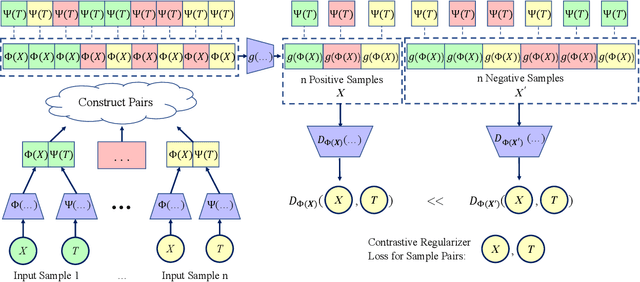
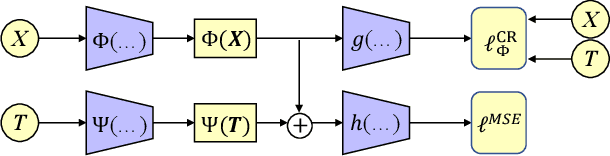
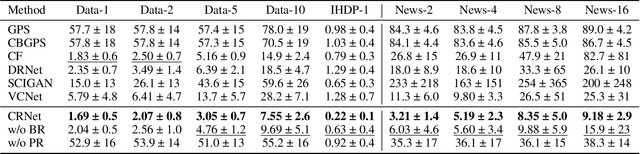
Abstract:Estimating the individuals' potential response to varying treatment doses is crucial for decision-making in areas such as precision medicine and management science. Most recent studies predict counterfactual outcomes by learning a covariate representation that is independent of the treatment variable. However, such independence constraints neglect much of the covariate information that is useful for counterfactual prediction, especially when the treatment variables are continuous. To tackle the above issue, in this paper, we first theoretically demonstrate the importance of the balancing and prognostic representations for unbiased estimation of the heterogeneous dose-response curves, that is, the learned representations are constrained to satisfy the conditional independence between the covariates and both of the treatment variables and the potential responses. Based on this, we propose a novel Contrastive balancing Representation learning Network using a partial distance measure, called CRNet, for estimating the heterogeneous dose-response curves without losing the continuity of treatments. Extensive experiments are conducted on synthetic and real-world datasets demonstrating that our proposal significantly outperforms previous methods.
Pareto-Optimal Estimation and Policy Learning on Short-term and Long-term Treatment Effects
Mar 12, 2024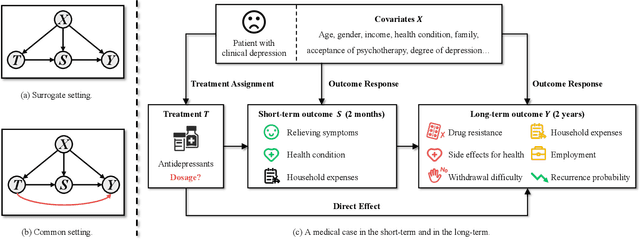



Abstract:This paper focuses on developing Pareto-optimal estimation and policy learning to identify the most effective treatment that maximizes the total reward from both short-term and long-term effects, which might conflict with each other. For example, a higher dosage of medication might increase the speed of a patient's recovery (short-term) but could also result in severe long-term side effects. Although recent works have investigated the problems about short-term or long-term effects or the both, how to trade-off between them to achieve optimal treatment remains an open challenge. Moreover, when multiple objectives are directly estimated using conventional causal representation learning, the optimization directions among various tasks can conflict as well. In this paper, we systematically investigate these issues and introduce a Pareto-Efficient algorithm, comprising Pareto-Optimal Estimation (POE) and Pareto-Optimal Policy Learning (POPL), to tackle them. POE incorporates a continuous Pareto module with representation balancing, enhancing estimation efficiency across multiple tasks. As for POPL, it involves deriving short-term and long-term outcomes linked with various treatment levels, facilitating an exploration of the Pareto frontier emanating from these outcomes. Results on both the synthetic and real-world datasets demonstrate the superiority of our method.
Instrumental Variables in Causal Inference and Machine Learning: A Survey
Dec 12, 2022Abstract:Causal inference is the process of using assumptions, study designs, and estimation strategies to draw conclusions about the causal relationships between variables based on data. This allows researchers to better understand the underlying mechanisms at work in complex systems and make more informed decisions. In many settings, we may not fully observe all the confounders that affect both the treatment and outcome variables, complicating the estimation of causal effects. To address this problem, a growing literature in both causal inference and machine learning proposes to use Instrumental Variables (IV). This paper serves as the first effort to systematically and comprehensively introduce and discuss the IV methods and their applications in both causal inference and machine learning. First, we provide the formal definition of IVs and discuss the identification problem of IV regression methods under different assumptions. Second, we categorize the existing work on IV methods into three streams according to the focus on the proposed methods, including two-stage least squares with IVs, control function with IVs, and evaluation of IVs. For each stream, we present both the classical causal inference methods, and recent developments in the machine learning literature. Then, we introduce a variety of applications of IV methods in real-world scenarios and provide a summary of the available datasets and algorithms. Finally, we summarize the literature, discuss the open problems and suggest promising future research directions for IV methods and their applications. We also develop a toolkit of IVs methods reviewed in this survey at https://github.com/causal-machine-learning-lab/mliv.
Confounder Balancing for Instrumental Variable Regression with Latent Variable
Nov 18, 2022Abstract:This paper studies the confounding effects from the unmeasured confounders and the imbalance of observed confounders in IV regression and aims at unbiased causal effect estimation. Recently, nonlinear IV estimators were proposed to allow for nonlinear model in both stages. However, the observed confounders may be imbalanced in stage 2, which could still lead to biased treatment effect estimation in certain cases. To this end, we propose a Confounder Balanced IV Regression (CB-IV) algorithm to jointly remove the bias from the unmeasured confounders and the imbalance of observed confounders. Theoretically, by redefining and solving an inverse problem for potential outcome function, we show that our CB-IV algorithm can unbiasedly estimate treatment effects and achieve lower variance. The IV methods have a major disadvantage in that little prior or theory is currently available to pre-define a valid IV in real-world scenarios. Thus, we study two more challenging settings without pre-defined valid IVs: (1) indistinguishable IVs implicitly present in observations, i.e., mixed-variable challenge, and (2) latent IVs don't appear in observations, i.e., latent-variable challenge. To address these two challenges, we extend our CB-IV by a latent-variable module, namely CB-IV-L algorithm. Extensive experiments demonstrate that our CB-IV(-L) outperforms the existing approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge