Ran Lu
Automated Olfactory Bulb Segmentation on High Resolutional T2-Weighted MRI
Aug 09, 2021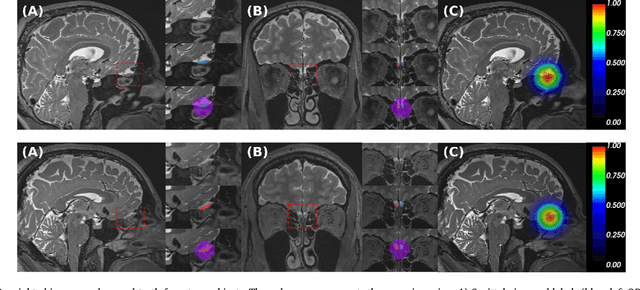

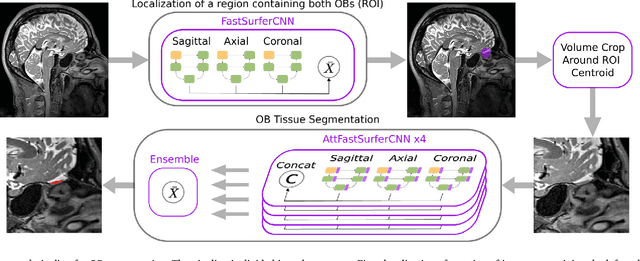
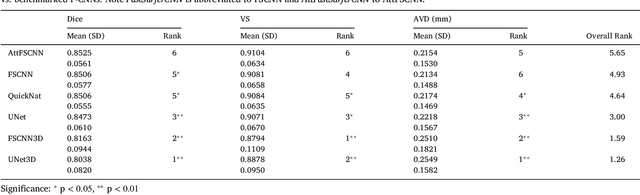
Abstract:The neuroimage analysis community has neglected the automated segmentation of the olfactory bulb (OB) despite its crucial role in olfactory function. The lack of an automatic processing method for the OB can be explained by its challenging properties. Nonetheless, recent advances in MRI acquisition techniques and resolution have allowed raters to generate more reliable manual annotations. Furthermore, the high accuracy of deep learning methods for solving semantic segmentation problems provides us with an option to reliably assess even small structures. In this work, we introduce a novel, fast, and fully automated deep learning pipeline to accurately segment OB tissue on sub-millimeter T2-weighted (T2w) whole-brain MR images. To this end, we designed a three-stage pipeline: (1) Localization of a region containing both OBs using FastSurferCNN, (2) Segmentation of OB tissue within the localized region through four independent AttFastSurferCNN - a novel deep learning architecture with a self-attention mechanism to improve modeling of contextual information, and (3) Ensemble of the predicted label maps. The OB pipeline exhibits high performance in terms of boundary delineation, OB localization, and volume estimation across a wide range of ages in 203 participants of the Rhineland Study. Moreover, it also generalizes to scans of an independent dataset never encountered during training, the Human Connectome Project (HCP), with different acquisition parameters and demographics, evaluated in 30 cases at the native 0.7mm HCP resolution, and the default 0.8mm pipeline resolution. We extensively validated our pipeline not only with respect to segmentation accuracy but also to known OB volume effects, where it can sensitively replicate age effects.
AxonEM Dataset: 3D Axon Instance Segmentation of Brain Cortical Regions
Jul 12, 2021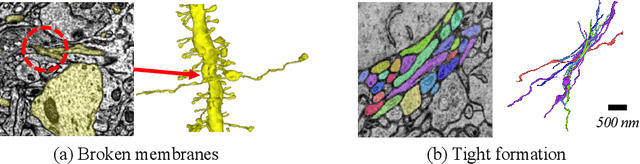

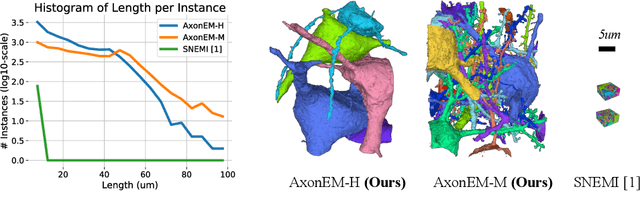
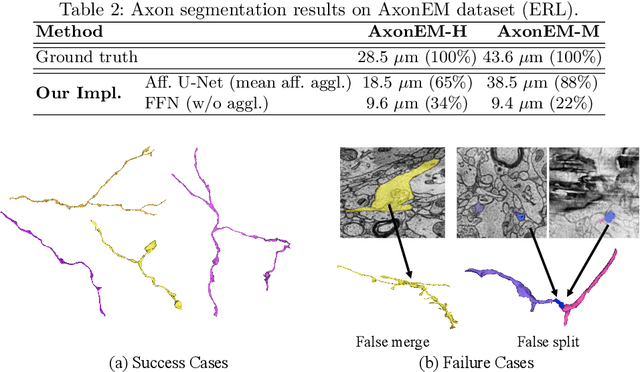
Abstract:Electron microscopy (EM) enables the reconstruction of neural circuits at the level of individual synapses, which has been transformative for scientific discoveries. However, due to the complex morphology, an accurate reconstruction of cortical axons has become a major challenge. Worse still, there is no publicly available large-scale EM dataset from the cortex that provides dense ground truth segmentation for axons, making it difficult to develop and evaluate large-scale axon reconstruction methods. To address this, we introduce the AxonEM dataset, which consists of two 30x30x30 um^3 EM image volumes from the human and mouse cortex, respectively. We thoroughly proofread over 18,000 axon instances to provide dense 3D axon instance segmentation, enabling large-scale evaluation of axon reconstruction methods. In addition, we densely annotate nine ground truth subvolumes for training, per each data volume. With this, we reproduce two published state-of-the-art methods and provide their evaluation results as a baseline. We publicly release our code and data at https://connectomics-bazaar.github.io/proj/AxonEM/index.html to foster the development of advanced methods.
Large-scale image segmentation based on distributed clustering algorithms
Jun 21, 2021



Abstract:Many approaches to 3D image segmentation are based on hierarchical clustering of supervoxels into image regions. Here we describe a distributed algorithm capable of handling a tremendous number of supervoxels. The algorithm works recursively, the regions are divided into chunks that are processed independently in parallel by multiple workers. At each round of the recursive procedure, the chunk size in all dimensions are doubled until a single chunk encompasses the entire image. The final result is provably independent of the chunking scheme, and the same as if the entire image were processed without division into chunks. This is nontrivial because a pair of adjacent regions is scored by some statistical property (e.g. mean or median) of the affinities at the interface, and the interface may extend over arbitrarily many chunks. The trick is to delay merge decisions for regions that touch chunk boundaries, and only complete them in a later round after the regions are fully contained within a chunk. We demonstrate the algorithm by clustering an affinity graph with over 1.5 trillion edges between 135 billion supervoxels derived from a 3D electron microscopic brain image.
Automated Neuron Shape Analysis from Electron Microscopy
May 29, 2020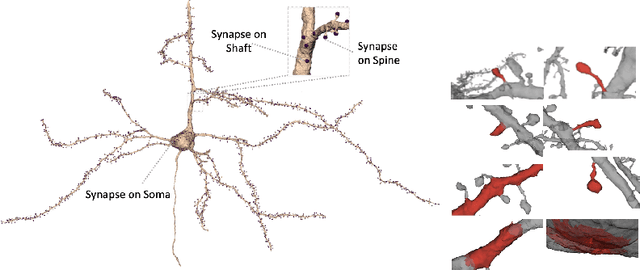
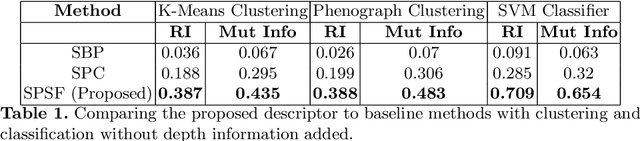
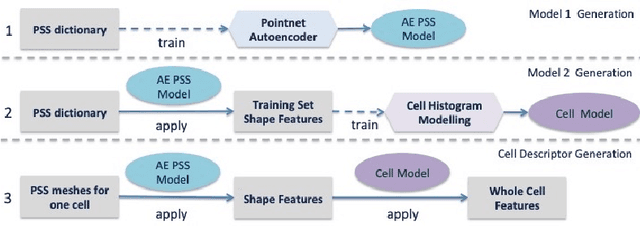
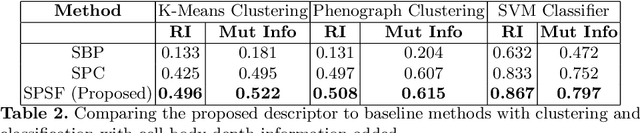
Abstract:Morphology based analysis of cell types has been an area of great interest to the neuroscience community for several decades. Recently, high resolution electron microscopy (EM) datasets of the mouse brain have opened up opportunities for data analysis at a level of detail that was previously impossible. These datasets are very large in nature and thus, manual analysis is not a practical solution. Of particular interest are details to the level of post synaptic structures. This paper proposes a fully automated framework for analysis of post-synaptic structure based neuron analysis from EM data. The processing framework involves shape extraction, representation with an autoencoder, and whole cell modeling and analysis based on shape distributions. We apply our novel framework on a dataset of 1031 neurons obtained from imaging a 1mm x 1mm x 40 micrometer volume of the mouse visual cortex and show the strength of our method in clustering and classification of neuronal shapes.
Learning Dense Voxel Embeddings for 3D Neuron Reconstruction
Sep 21, 2019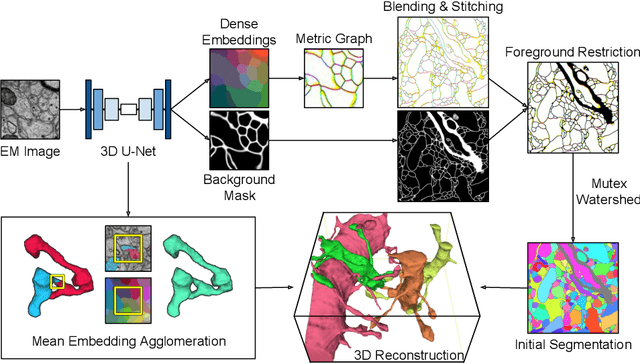
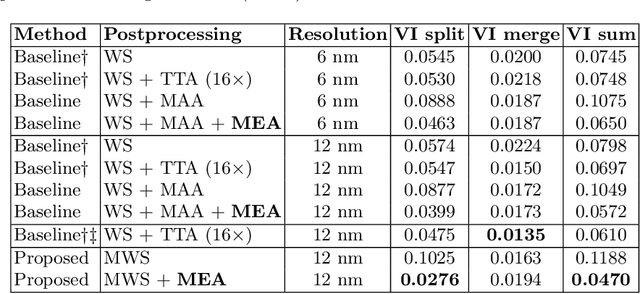
Abstract:We show dense voxel embeddings learned via deep metric learning can be employed to produce a highly accurate segmentation of neurons from 3D electron microscopy images. A metric graph on an arbitrary set of short and long-range edges can be constructed from the dense embeddings generated by a convolutional network. Partitioning the metric graph with long-range affinities as repulsive constraints can produce an initial segmentation with high precision, with substantial improvements on very thin objects. The convolutional embedding net is reused without any modification to agglomerate the systematic splits caused by complex "self-touching"' objects. Our proposed method achieves state-of-the-art accuracy on the challenging problem of 3D neuron reconstruction from the brain images acquired by serial section electron microscopy. Our alternative, object-centered representation could be more generally useful for other computational tasks in automated neural circuit reconstruction.
Convolutional nets for reconstructing neural circuits from brain images acquired by serial section electron microscopy
Apr 29, 2019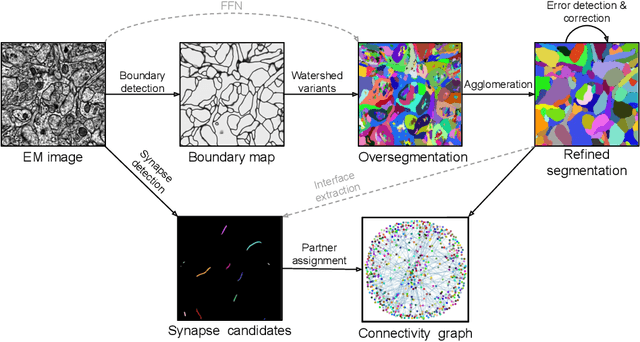

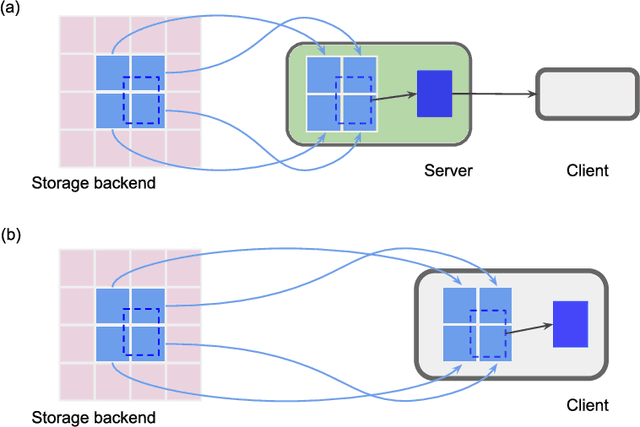
Abstract:Neural circuits can be reconstructed from brain images acquired by serial section electron microscopy. Image analysis has been performed by manual labor for half a century, and efforts at automation date back almost as far. Convolutional nets were first applied to neuronal boundary detection a dozen years ago, and have now achieved impressive accuracy on clean images. Robust handling of image defects is a major outstanding challenge. Convolutional nets are also being employed for other tasks in neural circuit reconstruction: finding synapses and identifying synaptic partners, extending or pruning neuronal reconstructions, and aligning serial section images to create a 3D image stack. Computational systems are being engineered to handle petavoxel images of cubic millimeter brain volumes.
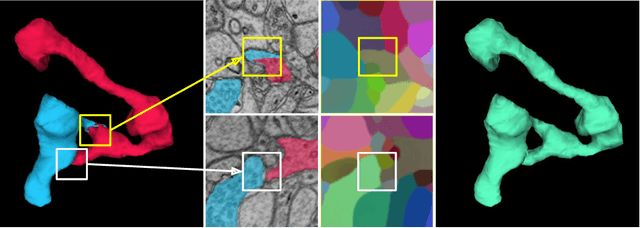
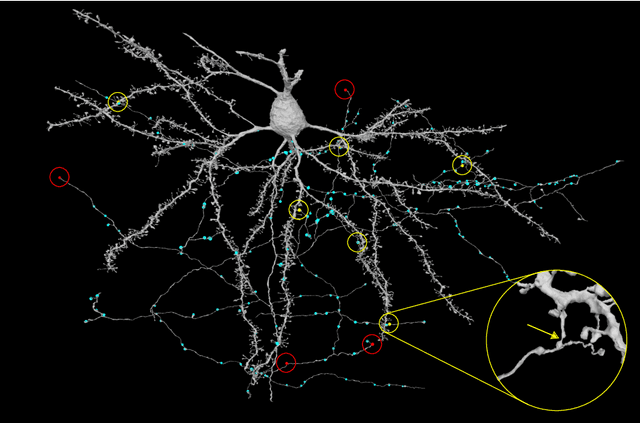
Synaptic Partner Assignment Using Attentional Voxel Association Networks
Apr 22, 2019

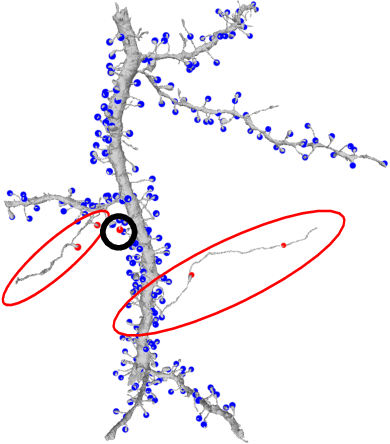
Abstract:Connectomics aims to recover a complete set of synaptic connections within a dataset imaged by electron microscopy. Most systems for locating synapses use voxelwise classifier models, and train these classifiers to reproduce binary masks of synaptic clefts. However, only recent work has included a way to identify the synaptic partners that communicate at synaptic cleft segments. Here, we present a novel method for associating synaptic cleft segments with their synaptic partners using a convolutional network trained to associate the mask of a cleft with the voxels of its synaptic partners. The network takes the local image context and a mask of a single cleft segment as input. It is trained to produce two volumes of output: one which labels the voxels of the presynaptic partner within the input image, and another similar volume for the postsynaptic partner. The cleft mask acts as an attentional gating signal for the network, in that two clefts with the same local image context often have different partners. We find that an implementation of this approach performs well on a dataset of mouse somatosensory cortex, and evaluate it as part of a combined system to predict both clefts and connections.
FatSegNet : A Fully Automated Deep Learning Pipeline for Adipose Tissue Segmentation on Abdominal Dixon MRI
Apr 03, 2019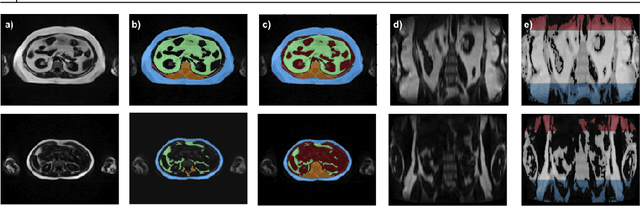



Abstract:Purpose: Development of a fast and fully automated deep learning pipeline (FatSegNet) to accurately identify, segment, and quantify abdominal adipose tissue on Dixon MRI from the Rhineland Study - a large prospective population-based study. Method: FatSegNet is composed of three stages: (i) consistent localization of the abdominal region using two 2D-Competitive Dense Fully Convolutional Networks (CDFNet), (ii) segmentation of adipose tissue on three views by independent CDFNets, and (iii) view aggregation. FatSegNet is trained with 33 manually annotated subjects, and validated by: 1) comparison of segmentation accuracy against a testingset covering a wide range of body mass index (BMI), 2) test-retest reliability, and 3) robustness in a large cohort study. Results: The CDFNet demonstrates increased robustness compared to traditional deep learning networks. FatSegNet dice score outperforms manual raters on the abdominal visceral adipose tissue (VAT, 0.828 vs. 0.788), and produces comparable results on subcutaneous adipose tissue (SAT, 0.973 vs. 0.982). The pipeline has very small test-retest absolute percentage difference and excellent agreement between scan sessions (VAT: APD = 2.957%, ICC=0.998 and SAT: APD= 3.254%, ICC=0.996). Conclusion: FatSegNet can reliably analyze a 3D Dixon MRI in1 min. It generalizes well to different body shapes, sensitively replicates known VAT and SAT volume effects in a large cohort study, and permits localized analysis of fat compartments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge