Prasanna Parvathaneni
Deep Learning Captures More Accurate Diffusion Fiber Orientations Distributions than Constrained Spherical Deconvolution
Nov 13, 2019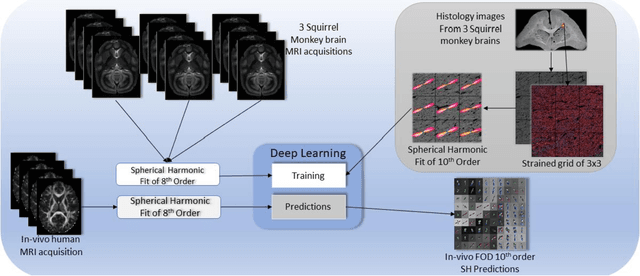
Abstract:Confocal histology provides an opportunity to establish intra-voxel fiber orientation distributions that can be used to quantitatively assess the biological relevance of diffusion weighted MRI models, e.g., constrained spherical deconvolution (CSD). Here, we apply deep learning to investigate the potential of single shell diffusion weighted MRI to explain histologically observed fiber orientation distributions (FOD) and compare the derived deep learning model with a leading CSD approach. This study (1) demonstrates that there exists additional information in the diffusion signal that is not currently exploited by CSD, and (2) provides an illustrative data-driven model that makes use of this information.
Enabling Multi-Shell b-Value Generalizability of Data-Driven Diffusion Models with Deep SHORE
Jul 15, 2019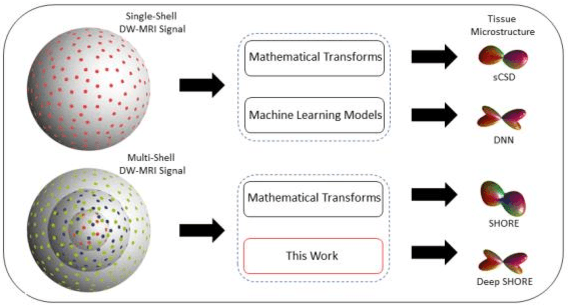
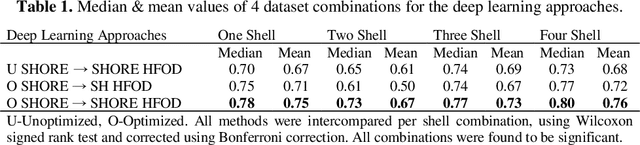
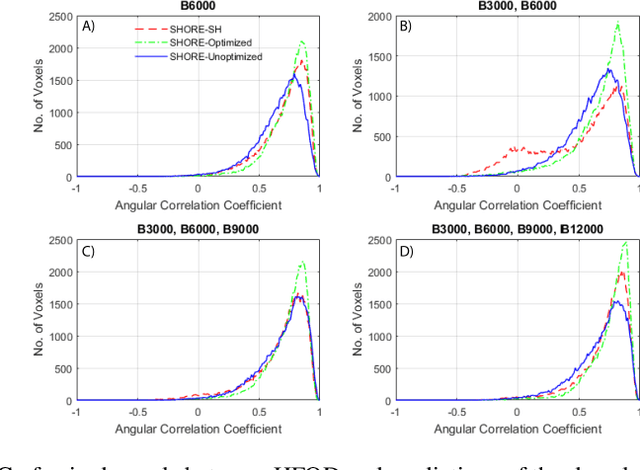
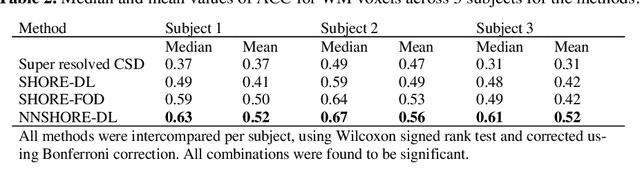
Abstract:Abstract. Intra-voxel models of the diffusion signal are essential for interpreting organization of the tissue environment at micrometer level with data at millimeter resolution. Recent advances in data driven methods have enabled direct compari-son and optimization of methods for in-vivo data with externally validated histological sections with both 2-D and 3-D histology. Yet, all existing methods make limiting assumptions of either (1) model-based linkages between b-values or (2) limited associations with single shell data. We generalize prior deep learning models that used single shell spherical harmonic transforms to integrate the re-cently developed simple harmonic oscillator reconstruction (SHORE) basis. To enable learning on the SHORE manifold, we present an alternative formulation of the fiber orientation distribution (FOD) object using the SHORE basis while rep-resenting the observed diffusion weighted data in the SHORE basis. To ensure consistency of hyper-parameter optimization for SHORE, we present our Deep SHORE approach to learn on a data-optimized manifold. Deep SHORE is evalu-ated with eight-fold cross-validation of a preclinical MRI-histology data with four b-values. Generalizability of in-vivo human data is evaluated on two separate 3T MRI scanners. Specificity in terms of angular correlation (ACC) with the preclinical data improved on single shell: 0.78 relative to 0.73 and 0.73, multi-shell: 0.80 relative to 0.74 (p < 0.001). In the in-vivo human data, Deep SHORE was more consistent across scanners with 0.63 relative to other multi-shell methods 0.39, 0.52 and 0.57 in terms of ACC. In conclusion, Deep SHORE is a promising method to enable data driven learning with DW-MRI under conditions with varying b-values, number of diffusion shells, and gradient directions per shell.
3D Whole Brain Segmentation using Spatially Localized Atlas Network Tiles
Mar 28, 2019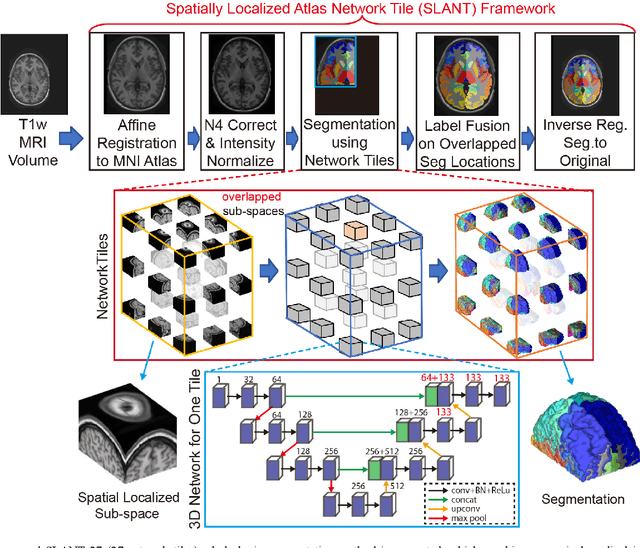
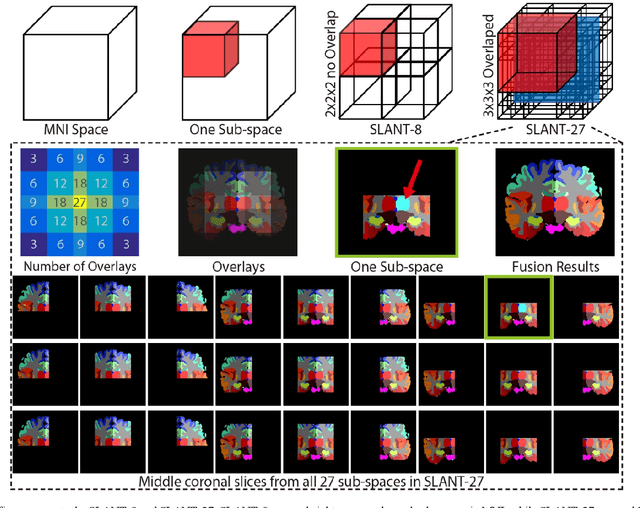
Abstract:Detailed whole brain segmentation is an essential quantitative technique, which provides a non-invasive way of measuring brain regions from a structural magnetic resonance imaging (MRI). Recently, deep convolution neural network (CNN) has been applied to whole brain segmentation. However, restricted by current GPU memory, 2D based methods, downsampling based 3D CNN methods, and patch-based high-resolution 3D CNN methods have been the de facto standard solutions. 3D patch-based high resolution methods typically yield superior performance among CNN approaches on detailed whole brain segmentation (>100 labels), however, whose performance are still commonly inferior compared with multi-atlas segmentation methods (MAS) due to the following challenges: (1) a single network is typically used to learn both spatial and contextual information for the patches, (2) limited manually traced whole brain volumes are available (typically less than 50) for training a network. In this work, we propose the spatially localized atlas network tiles (SLANT) method to distribute multiple independent 3D fully convolutional networks (FCN) for high-resolution whole brain segmentation. To address the first challenge, multiple spatially distributed networks were used in the SLANT method, in which each network learned contextual information for a fixed spatial location. To address the second challenge, auxiliary labels on 5111 initially unlabeled scans were created by multi-atlas segmentation for training. Since the method integrated multiple traditional medical image processing methods with deep learning, we developed a containerized pipeline to deploy the end-to-end solution. From the results, the proposed method achieved superior performance compared with multi-atlas segmentation methods, while reducing the computational time from >30 hours to 15 minutes (https://github.com/MASILab/SLANTbrainSeg).
Coronary Calcium Detection using 3D Attention Identical Dual Deep Network Based on Weakly Supervised Learning
Nov 10, 2018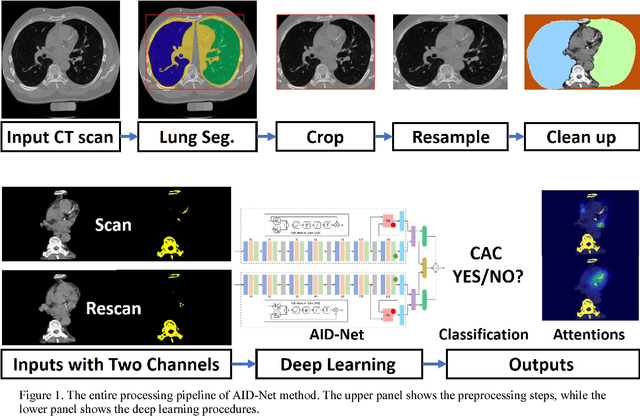
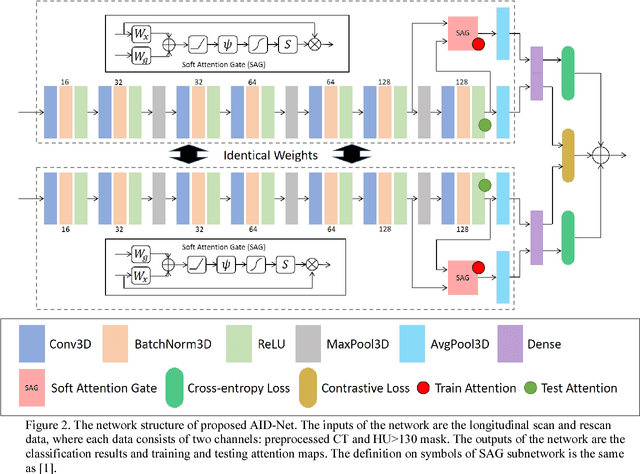

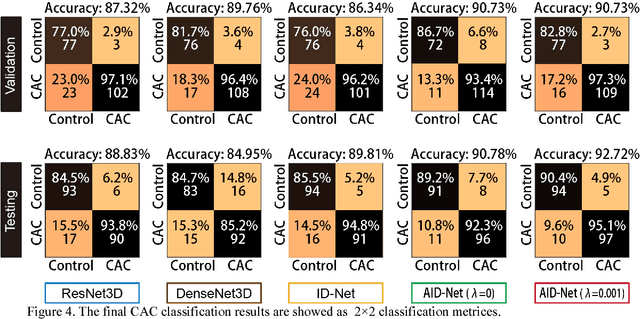
Abstract:Coronary artery calcium (CAC) is biomarker of advanced subclinical coronary artery disease and predicts myocardial infarction and death prior to age 60 years. The slice-wise manual delineation has been regarded as the gold standard of coronary calcium detection. However, manual efforts are time and resource consuming and even impracticable to be applied on large-scale cohorts. In this paper, we propose the attention identical dual network (AID-Net) to perform CAC detection using scan-rescan longitudinal non-contrast CT scans with weakly supervised attention by only using per scan level labels. To leverage the performance, 3D attention mechanisms were integrated into the AID-Net to provide complementary information for classification tasks. Moreover, the 3D Gradient-weighted Class Activation Mapping (Grad-CAM) was also proposed at the testing stage to interpret the behaviors of the deep neural network. 5075 non-contrast chest CT scans were used as training, validation and testing datasets. Baseline performance was assessed on the same cohort. From the results, the proposed AID-Net achieved the superior performance on classification accuracy (0.9272) and AUC (0.9627).
Splenomegaly Segmentation on Multi-modal MRI using Deep Convolutional Networks
Nov 09, 2018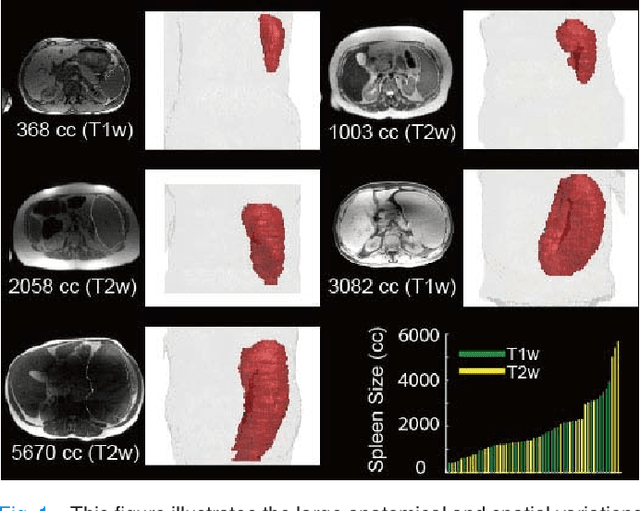
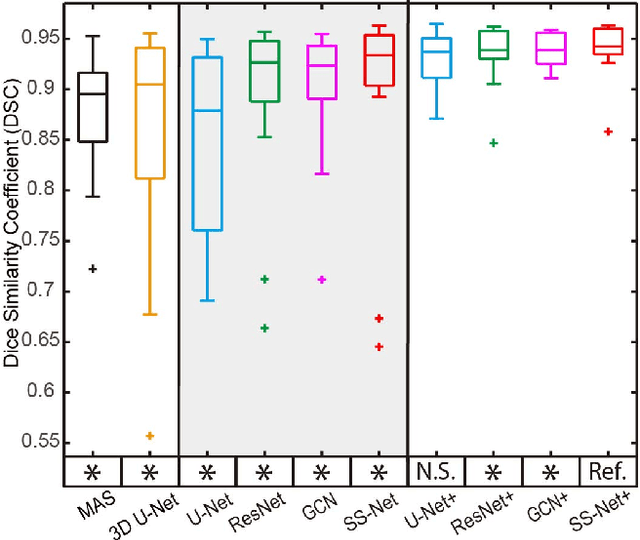
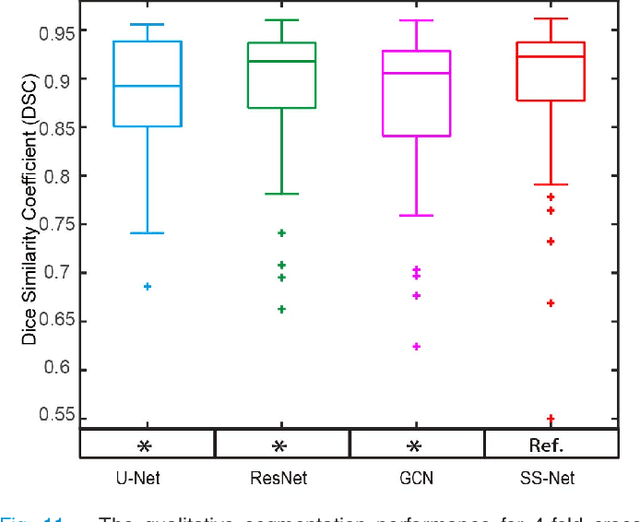
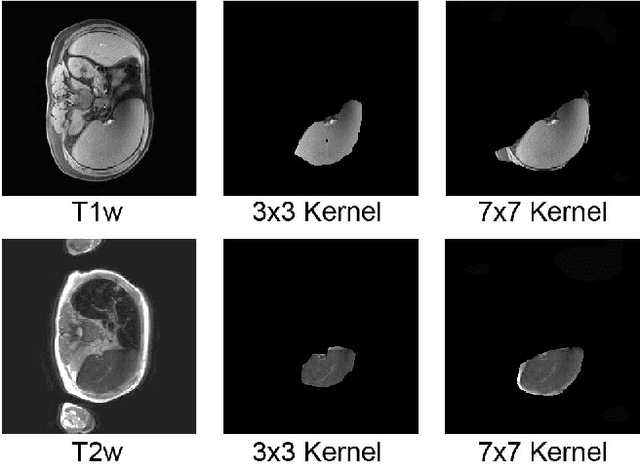
Abstract:The findings of splenomegaly, abnormal enlargement of the spleen, is a non-invasive clinical biomarker for liver and spleen disease. Automated segmentation methods are essential to efficiently quantify splenomegaly from clinically acquired abdominal magnetic resonance imaging (MRI) scans. However, the task is challenging due to (1) large anatomical and spatial variations of splenomegaly, (2) large inter- and intra-scan intensity variations on multi-modal MRI, and (3) limited numbers of labeled splenomegaly scans. In this paper, we propose the Splenomegaly Segmentation Network (SS-Net) to introduce the deep convolutional neural network (DCNN) approaches in multi-modal MRI splenomegaly segmentation. Large convolutional kernel layers were used to address the spatial and anatomical variations, while the conditional generative adversarial networks (GAN) were employed to leverage the segmentation performance of SS-Net in an end-to-end manner. A clinically acquired cohort containing both T1-weighted (T1w) and T2-weighted (T2w) MRI splenomegaly scans was used to train and evaluate the performance of multi-atlas segmentation (MAS), 2D DCNN networks, and a 3D DCNN network. From the experimental results, the DCNN methods achieved superior performance to the state-of-the-art MAS method. The proposed SS-Net method achieved the highest median and mean Dice scores among investigated baseline DCNN methods.
Inter-Scanner Harmonization of High Angular Resolution DW-MRI using Null Space Deep Learning
Oct 09, 2018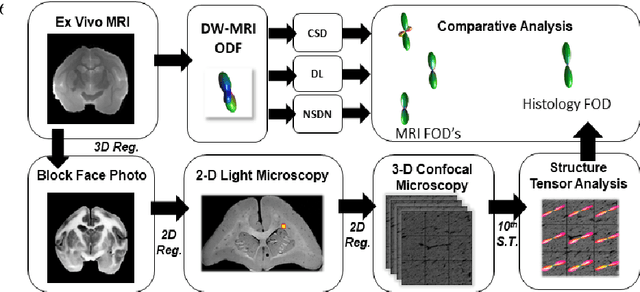
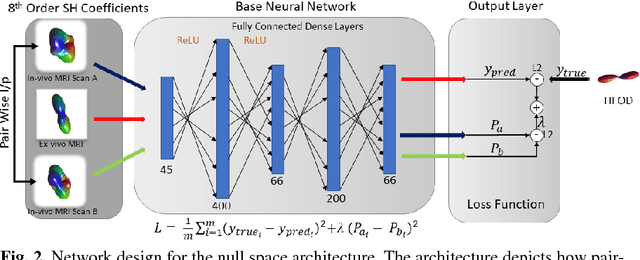
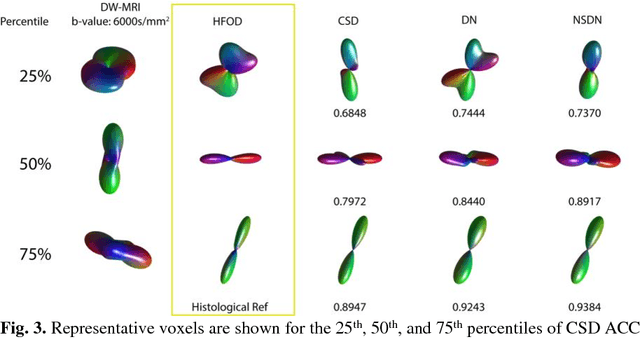
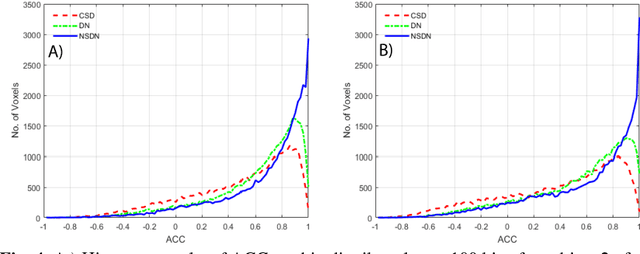
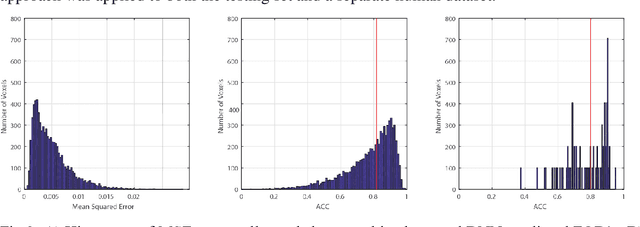
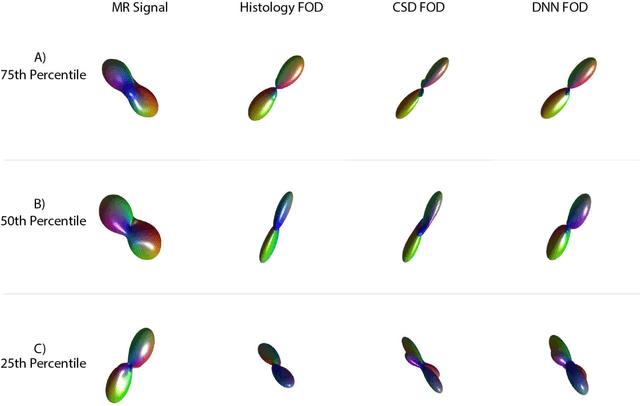
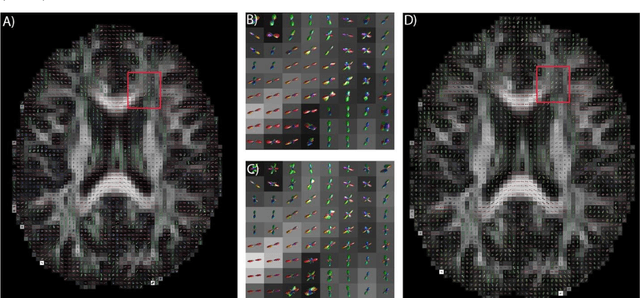
Abstract:Diffusion-weighted magnetic resonance imaging (DW-MRI) allows for non-invasive imaging of the local fiber architecture of the human brain at a millimetric scale. Multiple classical approaches have been proposed to detect both single (e.g., tensors) and multiple (e.g., constrained spherical deconvolution, CSD) fiber population orientations per voxel. However, existing techniques generally exhibit low reproducibility across MRI scanners. Herein, we propose a data-driven tech-nique using a neural network design which exploits two categories of data. First, training data were acquired on three squirrel monkey brains using ex-vivo DW-MRI and histology of the brain. Second, repeated scans of human subjects were acquired on two different scanners to augment the learning of the network pro-posed. To use these data, we propose a new network architecture, the null space deep network (NSDN), to simultaneously learn on traditional observed/truth pairs (e.g., MRI-histology voxels) along with repeated observations without a known truth (e.g., scan-rescan MRI). The NSDN was tested on twenty percent of the histology voxels that were kept completely blind to the network. NSDN significantly improved absolute performance relative to histology by 3.87% over CSD and 1.42% over a recently proposed deep neural network approach. More-over, it improved reproducibility on the paired data by 21.19% over CSD and 10.09% over a recently proposed deep approach. Finally, NSDN improved gen-eralizability of the model to a third in vivo human scanner (which was not used in training) by 16.08% over CSD and 10.41% over a recently proposed deep learn-ing approach. This work suggests that data-driven approaches for local fiber re-construction are more reproducible, informative and precise and offers a novel, practical method for determining these models.
Spatially Localized Atlas Network Tiles Enables 3D Whole Brain Segmentation from Limited Data
Jun 05, 2018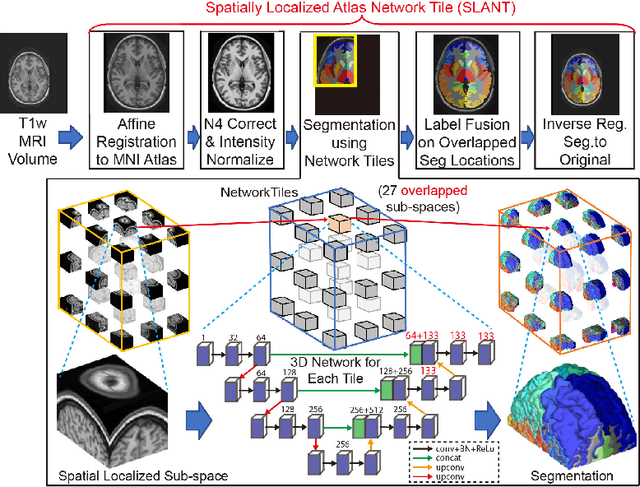
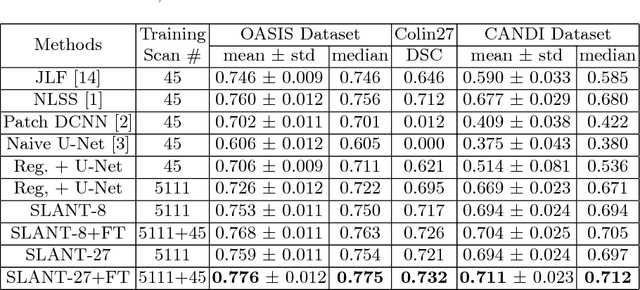
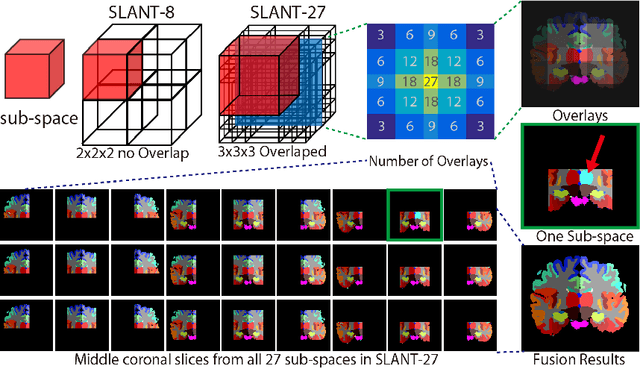
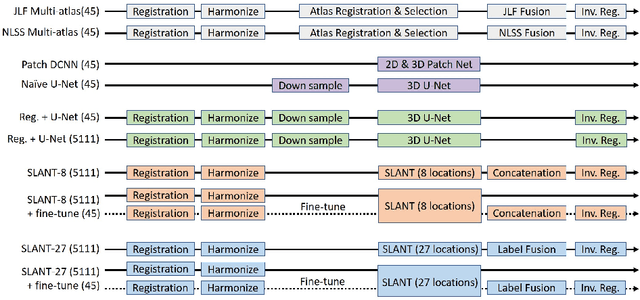
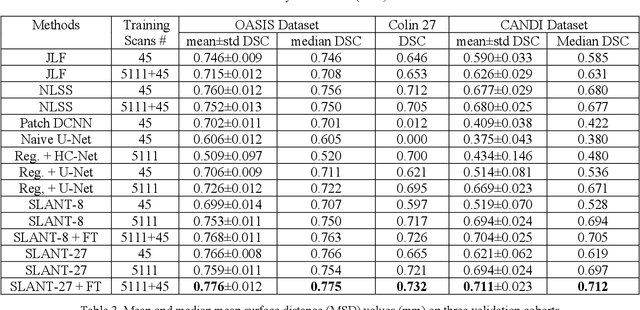
Abstract:Whole brain segmentation on a structural magnetic resonance imaging (MRI) is essential in non-invasive investigation for neuroanatomy. Historically, multi-atlas segmentation (MAS) has been regarded as the de facto standard method for whole brain segmentation. Recently, deep neural network approaches have been applied to whole brain segmentation by learning random patches or 2D slices. Yet, few previous efforts have been made on detailed whole brain segmentation using 3D networks due to the following challenges: (1) fitting entire whole brain volume into 3D networks is restricted by the current GPU memory, and (2) the large number of targeting labels (e.g., > 100 labels) with limited number of training 3D volumes (e.g., < 50 scans). In this paper, we propose the spatially localized atlas network tiles (SLANT) method to distribute multiple independent 3D fully convolutional networks to cover overlapped sub-spaces in a standard atlas space. This strategy simplifies the whole brain learning task to localized sub-tasks, which was enabled by combing canonical registration and label fusion techniques with deep learning. To address the second challenge, auxiliary labels on 5111 initially unlabeled scans were created by MAS for pre-training. From empirical validation, the state-of-the-art MAS method achieved mean Dice value of 0.76, 0.71, and 0.68, while the proposed method achieved 0.78, 0.73, and 0.71 on three validation cohorts. Moreover, the computational time reduced from > 30 hours using MAS to ~15 minutes using the proposed method. The source code is available online https://github.com/MASILab/SLANTbrainSeg
Improved Stability of Whole Brain Surface Parcellation with Multi-Atlas Segmentation
Dec 02, 2017Abstract:Whole brain segmentation and cortical surface parcellation are essential in understanding the anatomical-functional relationships of the brain. Multi-atlas segmentation has been regarded as one of the leading segmentation methods for the whole brain segmentation. In our recent work, the multi-atlas technique has been adapted to surface reconstruction using a method called Multi-atlas CRUISE (MaCRUISE). The MaCRUISE method not only performed consistent volume-surface analyses but also showed advantages on robustness compared with the FreeSurfer method. However, a detailed surface parcellation was not provided by MaCRUISE, which hindered the region of interest (ROI) based analyses on surfaces. Herein, the MaCRUISE surface parcellation (MaCRUISEsp) method is proposed to perform the surface parcellation upon the inner, central and outer surfaces that are reconstructed from MaCRUISE. MaCRUISEsp parcellates inner, central and outer surfaces with 98 cortical labels respectively using a volume segmentation based surface parcellation (VSBSP), following a topological correction step. To validate the performance of MaCRUISEsp, 21 scan-rescan magnetic resonance imaging (MRI) T1 volume pairs from the Kirby21 dataset were used to perform a reproducibility analyses. MaCRUISEsp achieved 0.948 on median Dice Similarity Coefficient (DSC) for central surfaces. Meanwhile, FreeSurfer achieved 0.905 DSC for inner surfaces and 0.881 DSC for outer surfaces, while the proposed method achieved 0.929 DSC for inner surfaces and 0.835 DSC for outer surfaces. Qualitatively, the results are encouraging, but are not directly comparable as the two approaches use different definitions of cortical labels.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge