Allison E. Hainline
Deep Learning Captures More Accurate Diffusion Fiber Orientations Distributions than Constrained Spherical Deconvolution
Nov 13, 2019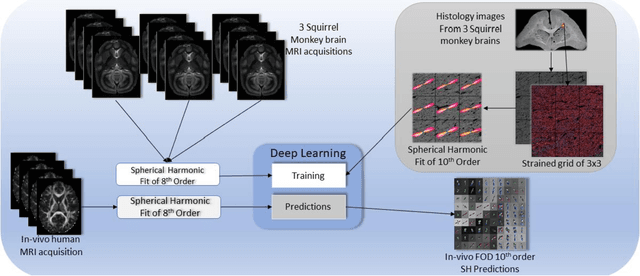
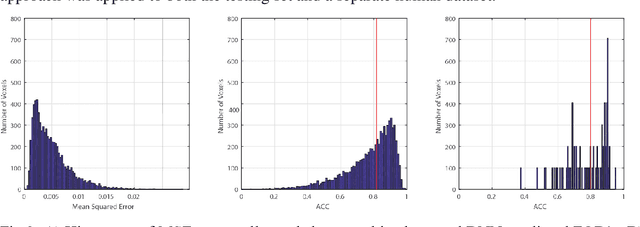
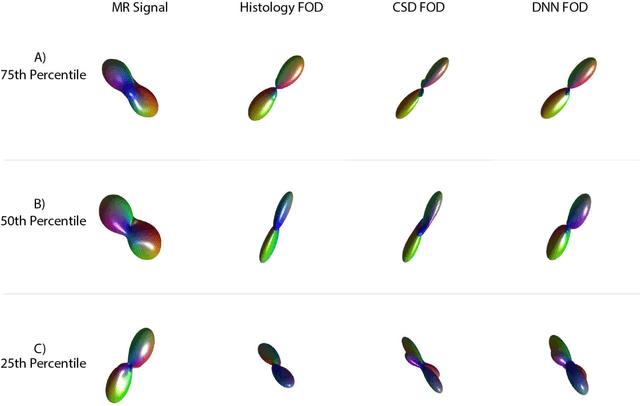
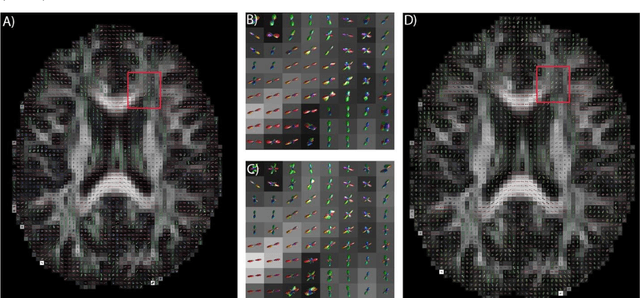
Abstract:Confocal histology provides an opportunity to establish intra-voxel fiber orientation distributions that can be used to quantitatively assess the biological relevance of diffusion weighted MRI models, e.g., constrained spherical deconvolution (CSD). Here, we apply deep learning to investigate the potential of single shell diffusion weighted MRI to explain histologically observed fiber orientation distributions (FOD) and compare the derived deep learning model with a leading CSD approach. This study (1) demonstrates that there exists additional information in the diffusion signal that is not currently exploited by CSD, and (2) provides an illustrative data-driven model that makes use of this information.
Towards Machine Learning Prediction of Deep Brain Stimulation (DBS) Intra-operative Efficacy Maps
Nov 26, 2018
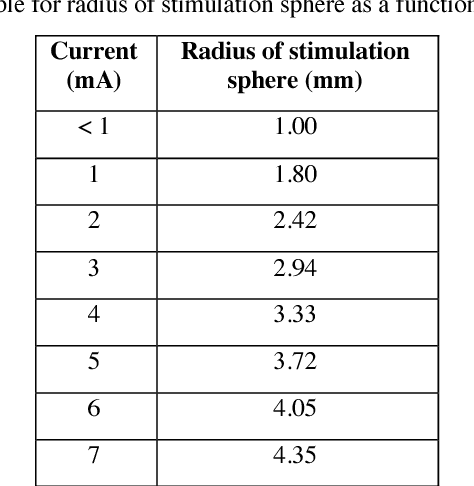


Abstract:Deep brain stimulation (DBS) has the potential to improve the quality of life of people with a variety of neurological diseases. A key challenge in DBS is in the placement of a stimulation electrode in the anatomical location that maximizes efficacy and minimizes side effects. Pre-operative localization of the optimal stimulation zone can reduce surgical times and morbidity. Current methods of producing efficacy probability maps follow an anatomical guidance on magnetic resonance imaging (MRI) to identify the areas with the highest efficacy in a population. In this work, we propose to revisit this problem as a classification problem, where each voxel in the MRI is a sample informed by the surrounding anatomy. We use a patch-based convolutional neural network to classify a stimulation coordinate as having a positive reduction in symptoms during surgery. We use a cohort of 187 patients with a total of 2,869 stimulation coordinates, upon which 3D patches were extracted and associated with an efficacy score. We compare our results with a registration-based method of surgical planning. We show an improvement in the classification of intraoperative stimulation coordinates as a positive response in reduction of symptoms with AUC of 0.670 compared to a baseline registration-based approach, which achieves an AUC of 0.627 (p < 0.01). Although additional validation is needed, the proposed classification framework and deep learning method appear well-suited for improving pre-surgical planning and personalize treatment strategies.
Inter-Scanner Harmonization of High Angular Resolution DW-MRI using Null Space Deep Learning
Oct 09, 2018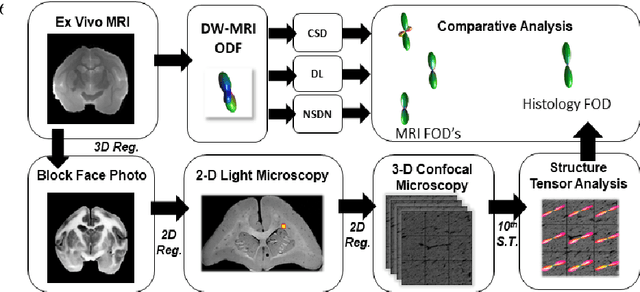
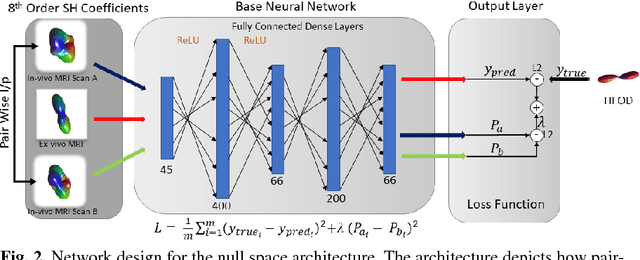
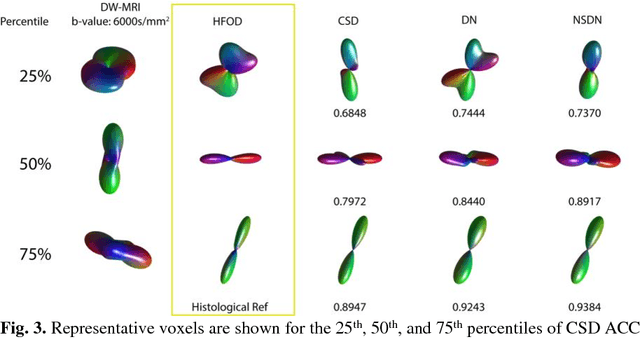
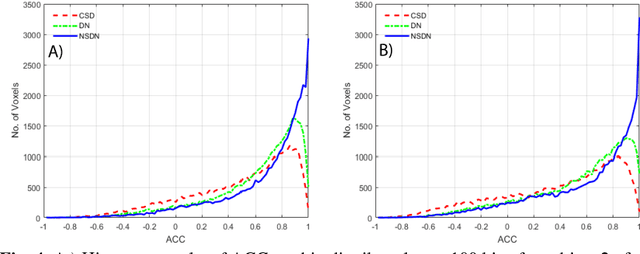
Abstract:Diffusion-weighted magnetic resonance imaging (DW-MRI) allows for non-invasive imaging of the local fiber architecture of the human brain at a millimetric scale. Multiple classical approaches have been proposed to detect both single (e.g., tensors) and multiple (e.g., constrained spherical deconvolution, CSD) fiber population orientations per voxel. However, existing techniques generally exhibit low reproducibility across MRI scanners. Herein, we propose a data-driven tech-nique using a neural network design which exploits two categories of data. First, training data were acquired on three squirrel monkey brains using ex-vivo DW-MRI and histology of the brain. Second, repeated scans of human subjects were acquired on two different scanners to augment the learning of the network pro-posed. To use these data, we propose a new network architecture, the null space deep network (NSDN), to simultaneously learn on traditional observed/truth pairs (e.g., MRI-histology voxels) along with repeated observations without a known truth (e.g., scan-rescan MRI). The NSDN was tested on twenty percent of the histology voxels that were kept completely blind to the network. NSDN significantly improved absolute performance relative to histology by 3.87% over CSD and 1.42% over a recently proposed deep neural network approach. More-over, it improved reproducibility on the paired data by 21.19% over CSD and 10.09% over a recently proposed deep approach. Finally, NSDN improved gen-eralizability of the model to a third in vivo human scanner (which was not used in training) by 16.08% over CSD and 10.41% over a recently proposed deep learn-ing approach. This work suggests that data-driven approaches for local fiber re-construction are more reproducible, informative and precise and offers a novel, practical method for determining these models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge