Andrew J. Plassard
Deep Learning Captures More Accurate Diffusion Fiber Orientations Distributions than Constrained Spherical Deconvolution
Nov 13, 2019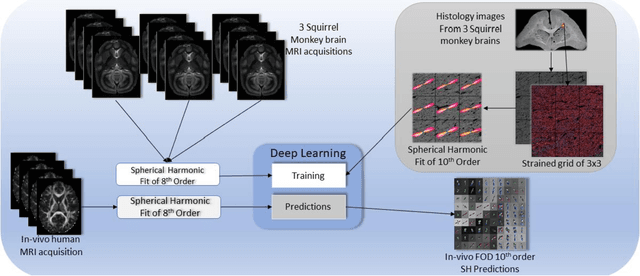
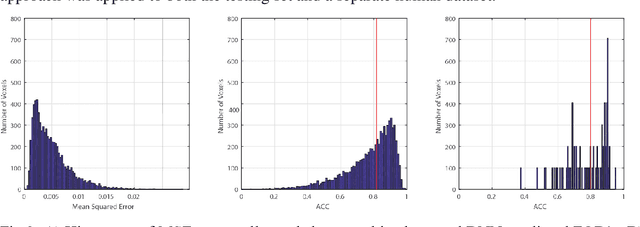
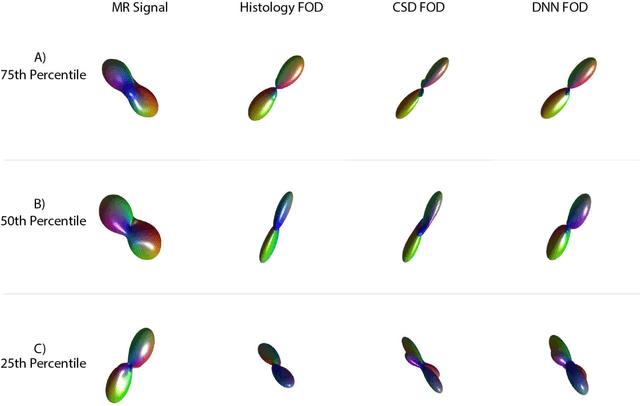
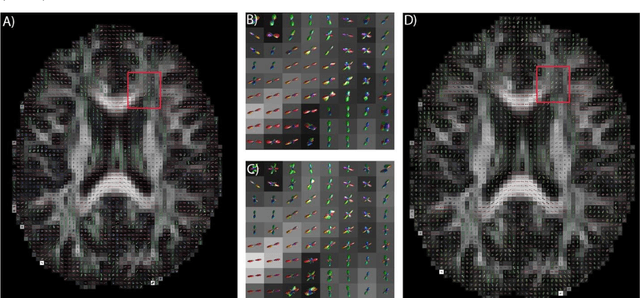
Abstract:Confocal histology provides an opportunity to establish intra-voxel fiber orientation distributions that can be used to quantitatively assess the biological relevance of diffusion weighted MRI models, e.g., constrained spherical deconvolution (CSD). Here, we apply deep learning to investigate the potential of single shell diffusion weighted MRI to explain histologically observed fiber orientation distributions (FOD) and compare the derived deep learning model with a leading CSD approach. This study (1) demonstrates that there exists additional information in the diffusion signal that is not currently exploited by CSD, and (2) provides an illustrative data-driven model that makes use of this information.
Learning Implicit Brain MRI Manifolds with Deep Learning
Jan 05, 2018Abstract:An important task in image processing and neuroimaging is to extract quantitative information from the acquired images in order to make observations about the presence of disease or markers of development in populations. Having a lowdimensional manifold of an image allows for easier statistical comparisons between groups and the synthesis of group representatives. Previous studies have sought to identify the best mapping of brain MRI to a low-dimensional manifold, but have been limited by assumptions of explicit similarity measures. In this work, we use deep learning techniques to investigate implicit manifolds of normal brains and generate new, high-quality images. We explore implicit manifolds by addressing the problems of image synthesis and image denoising as important tools in manifold learning. First, we propose the unsupervised synthesis of T1-weighted brain MRI using a Generative Adversarial Network (GAN) by learning from 528 examples of 2D axial slices of brain MRI. Synthesized images were first shown to be unique by performing a crosscorrelation with the training set. Real and synthesized images were then assessed in a blinded manner by two imaging experts providing an image quality score of 1-5. The quality score of the synthetic image showed substantial overlap with that of the real images. Moreover, we use an autoencoder with skip connections for image denoising, showing that the proposed method results in higher PSNR than FSL SUSAN after denoising. This work shows the power of artificial networks to synthesize realistic imaging data, which can be used to improve image processing techniques and provide a quantitative framework to structural changes in the brain.
Splenomegaly Segmentation using Global Convolutional Kernels and Conditional Generative Adversarial Networks
Dec 02, 2017Abstract:Spleen volume estimation using automated image segmentation technique may be used to detect splenomegaly (abnormally enlarged spleen) on Magnetic Resonance Imaging (MRI) scans. In recent years, Deep Convolutional Neural Networks (DCNN) segmentation methods have demonstrated advantages for abdominal organ segmentation. However, variations in both size and shape of the spleen on MRI images may result in large false positive and false negative labeling when deploying DCNN based methods. In this paper, we propose the Splenomegaly Segmentation Network (SSNet) to address spatial variations when segmenting extraordinarily large spleens. SSNet was designed based on the framework of image-to-image conditional generative adversarial networks (cGAN). Specifically, the Global Convolutional Network (GCN) was used as the generator to reduce false negatives, while the Markovian discriminator (PatchGAN) was used to alleviate false positives. A cohort of clinically acquired 3D MRI scans (both T1 weighted and T2 weighted) from patients with splenomegaly were used to train and test the networks. The experimental results demonstrated that a mean Dice coefficient of 0.9260 and a median Dice coefficient of 0.9262 using SSNet on independently tested MRI volumes of patients with splenomegaly.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge