Lisa C. Adams
RSNA Large Language Model Benchmark Dataset for Chest Radiographs of Cardiothoracic Disease: Radiologist Evaluation and Validation Enhanced by AI Labels (REVEAL-CXR)
Jan 21, 2026Abstract:Multimodal large language models have demonstrated comparable performance to that of radiology trainees on multiple-choice board-style exams. However, to develop clinically useful multimodal LLM tools, high-quality benchmarks curated by domain experts are essential. To curate released and holdout datasets of 100 chest radiographic studies each and propose an artificial intelligence (AI)-assisted expert labeling procedure to allow radiologists to label studies more efficiently. A total of 13,735 deidentified chest radiographs and their corresponding reports from the MIDRC were used. GPT-4o extracted abnormal findings from the reports, which were then mapped to 12 benchmark labels with a locally hosted LLM (Phi-4-Reasoning). From these studies, 1,000 were sampled on the basis of the AI-suggested benchmark labels for expert review; the sampling algorithm ensured that the selected studies were clinically relevant and captured a range of difficulty levels. Seventeen chest radiologists participated, and they marked "Agree all", "Agree mostly" or "Disagree" to indicate their assessment of the correctness of the LLM suggested labels. Each chest radiograph was evaluated by three experts. Of these, at least two radiologists selected "Agree All" for 381 radiographs. From this set, 200 were selected, prioritizing those with less common or multiple finding labels, and divided into 100 released radiographs and 100 reserved as the holdout dataset. The holdout dataset is used exclusively by RSNA to independently evaluate different models. A benchmark of 200 chest radiographic studies with 12 benchmark labels was created and made publicly available https://imaging.rsna.org, with each chest radiograph verified by three radiologists. In addition, an AI-assisted labeling procedure was developed to help radiologists label at scale, minimize unnecessary omissions, and support a semicollaborative environment.
Improving Reliability and Explainability of Medical Question Answering through Atomic Fact Checking in Retrieval-Augmented LLMs
May 30, 2025Abstract:Large language models (LLMs) exhibit extensive medical knowledge but are prone to hallucinations and inaccurate citations, which pose a challenge to their clinical adoption and regulatory compliance. Current methods, such as Retrieval Augmented Generation, partially address these issues by grounding answers in source documents, but hallucinations and low fact-level explainability persist. In this work, we introduce a novel atomic fact-checking framework designed to enhance the reliability and explainability of LLMs used in medical long-form question answering. This method decomposes LLM-generated responses into discrete, verifiable units called atomic facts, each of which is independently verified against an authoritative knowledge base of medical guidelines. This approach enables targeted correction of errors and direct tracing to source literature, thereby improving the factual accuracy and explainability of medical Q&A. Extensive evaluation using multi-reader assessments by medical experts and an automated open Q&A benchmark demonstrated significant improvements in factual accuracy and explainability. Our framework achieved up to a 40% overall answer improvement and a 50% hallucination detection rate. The ability to trace each atomic fact back to the most relevant chunks from the database provides a granular, transparent explanation of the generated responses, addressing a major gap in current medical AI applications. This work represents a crucial step towards more trustworthy and reliable clinical applications of LLMs, addressing key prerequisites for clinical application and fostering greater confidence in AI-assisted healthcare.
Robust Kidney Abnormality Segmentation: A Validation Study of an AI-Based Framework
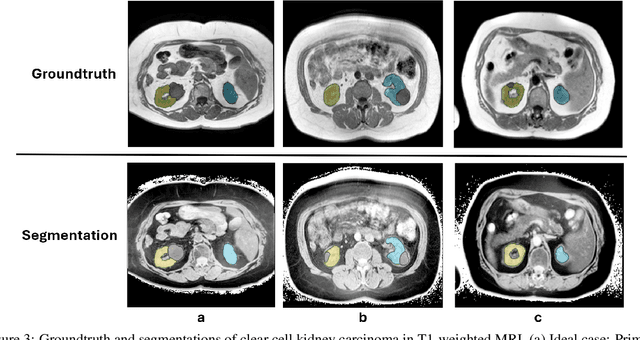
May 12, 2025Abstract:Kidney abnormality segmentation has important potential to enhance the clinical workflow, especially in settings requiring quantitative assessments. Kidney volume could serve as an important biomarker for renal diseases, with changes in volume correlating directly with kidney function. Currently, clinical practice often relies on subjective visual assessment for evaluating kidney size and abnormalities, including tumors and cysts, which are typically staged based on diameter, volume, and anatomical location. To support a more objective and reproducible approach, this research aims to develop a robust, thoroughly validated kidney abnormality segmentation algorithm, made publicly available for clinical and research use. We employ publicly available training datasets and leverage the state-of-the-art medical image segmentation framework nnU-Net. Validation is conducted using both proprietary and public test datasets, with segmentation performance quantified by Dice coefficient and the 95th percentile Hausdorff distance. Furthermore, we analyze robustness across subgroups based on patient sex, age, CT contrast phases, and tumor histologic subtypes. Our findings demonstrate that our segmentation algorithm, trained exclusively on publicly available data, generalizes effectively to external test sets and outperforms existing state-of-the-art models across all tested datasets. Subgroup analyses reveal consistent high performance, indicating strong robustness and reliability. The developed algorithm and associated code are publicly accessible at https://github.com/DIAGNijmegen/oncology-kidney-abnormality-segmentation.
Biomedical Large Languages Models Seem not to be Superior to Generalist Models on Unseen Medical Data
Aug 25, 2024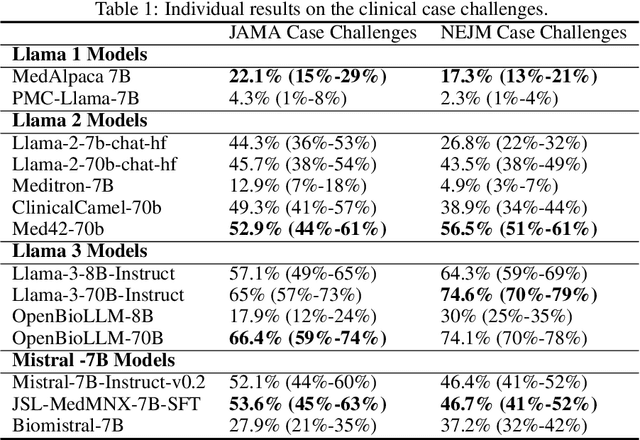
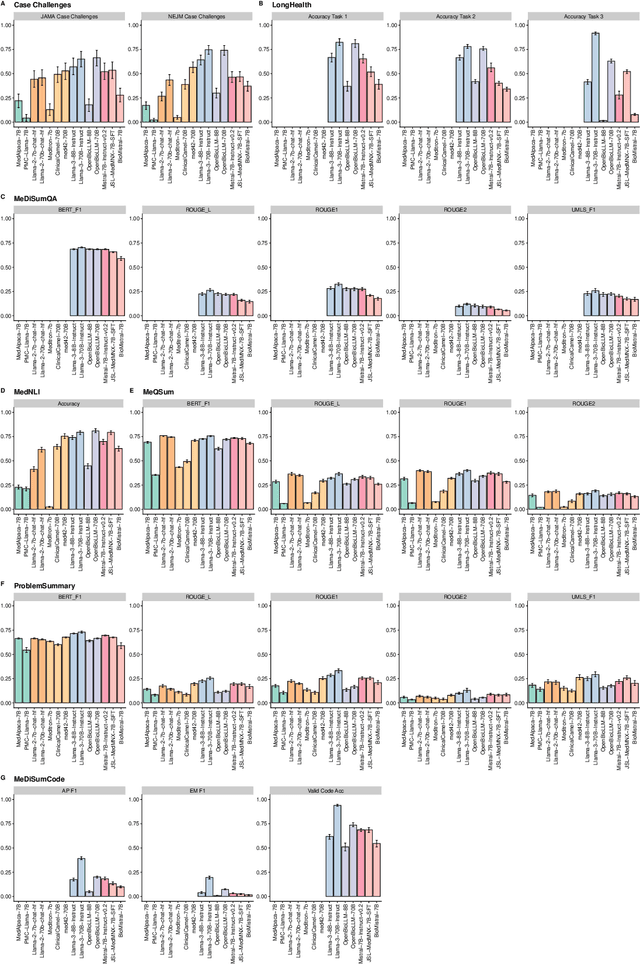
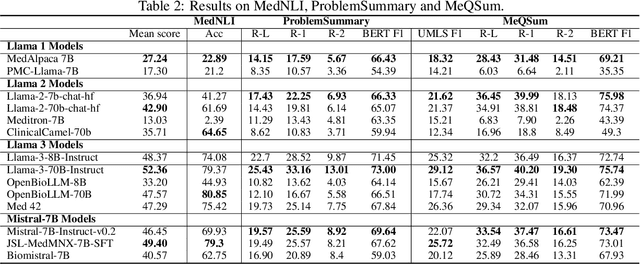
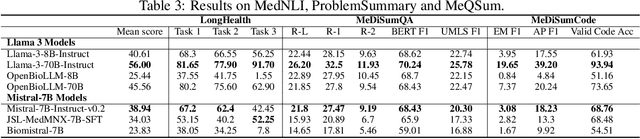
Abstract:Large language models (LLMs) have shown potential in biomedical applications, leading to efforts to fine-tune them on domain-specific data. However, the effectiveness of this approach remains unclear. This study evaluates the performance of biomedically fine-tuned LLMs against their general-purpose counterparts on a variety of clinical tasks. We evaluated their performance on clinical case challenges from the New England Journal of Medicine (NEJM) and the Journal of the American Medical Association (JAMA) and on several clinical tasks (e.g., information extraction, document summarization, and clinical coding). Using benchmarks specifically chosen to be likely outside the fine-tuning datasets of biomedical models, we found that biomedical LLMs mostly perform inferior to their general-purpose counterparts, especially on tasks not focused on medical knowledge. While larger models showed similar performance on case tasks (e.g., OpenBioLLM-70B: 66.4% vs. Llama-3-70B-Instruct: 65% on JAMA cases), smaller biomedical models showed more pronounced underperformance (e.g., OpenBioLLM-8B: 30% vs. Llama-3-8B-Instruct: 64.3% on NEJM cases). Similar trends were observed across the CLUE (Clinical Language Understanding Evaluation) benchmark tasks, with general-purpose models often performing better on text generation, question answering, and coding tasks. Our results suggest that fine-tuning LLMs to biomedical data may not provide the expected benefits and may potentially lead to reduced performance, challenging prevailing assumptions about domain-specific adaptation of LLMs and highlighting the need for more rigorous evaluation frameworks in healthcare AI. Alternative approaches, such as retrieval-augmented generation, may be more effective in enhancing the biomedical capabilities of LLMs without compromising their general knowledge.
Incorporating Anatomical Awareness for Enhanced Generalizability and Progression Prediction in Deep Learning-Based Radiographic Sacroiliitis Detection
May 12, 2024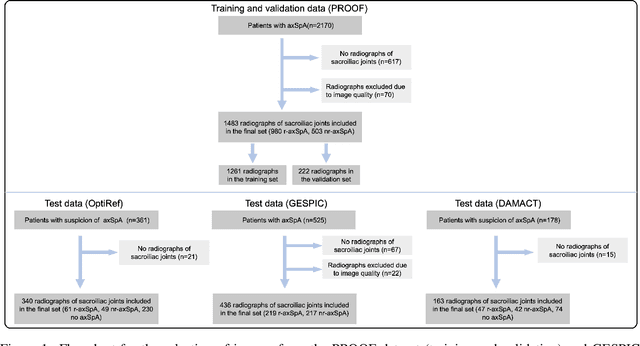
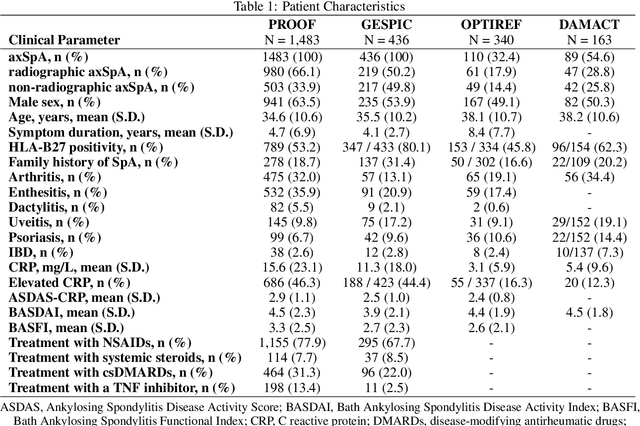
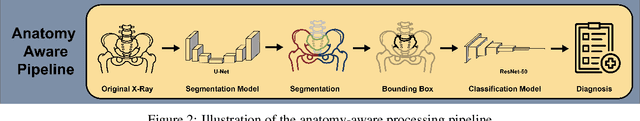

Abstract:Purpose: To examine whether incorporating anatomical awareness into a deep learning model can improve generalizability and enable prediction of disease progression. Methods: This retrospective multicenter study included conventional pelvic radiographs of 4 different patient cohorts focusing on axial spondyloarthritis (axSpA) collected at university and community hospitals. The first cohort, which consisted of 1483 radiographs, was split into training (n=1261) and validation (n=222) sets. The other cohorts comprising 436, 340, and 163 patients, respectively, were used as independent test datasets. For the second cohort, follow-up data of 311 patients was used to examine progression prediction capabilities. Two neural networks were trained, one on images cropped to the bounding box of the sacroiliac joints (anatomy-aware) and the other one on full radiographs. The performance of the models was compared using the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, and specificity. Results: On the three test datasets, the standard model achieved AUC scores of 0.853, 0.817, 0.947, with an accuracy of 0.770, 0.724, 0.850. Whereas the anatomy-aware model achieved AUC scores of 0.899, 0.846, 0.957, with an accuracy of 0.821, 0.744, 0.906, respectively. The patients who were identified as high risk by the anatomy aware model had an odds ratio of 2.16 (95% CI: 1.19, 3.86) for having progression of radiographic sacroiliitis within 2 years. Conclusion: Anatomical awareness can improve the generalizability of a deep learning model in detecting radiographic sacroiliitis. The model is published as fully open source alongside this study.
MRSegmentator: Robust Multi-Modality Segmentation of 40 Classes in MRI and CT Sequences
May 10, 2024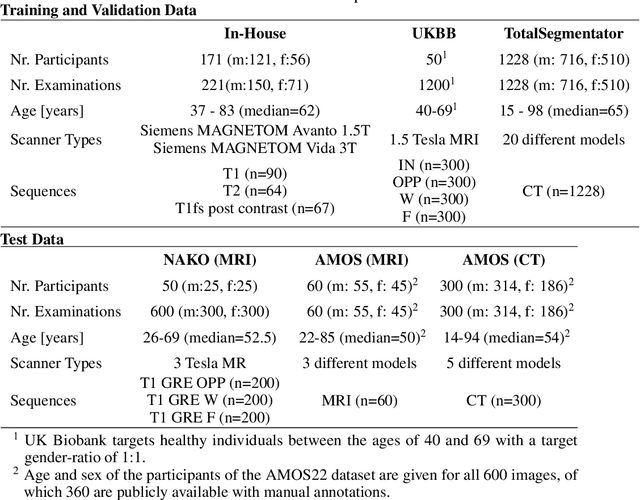
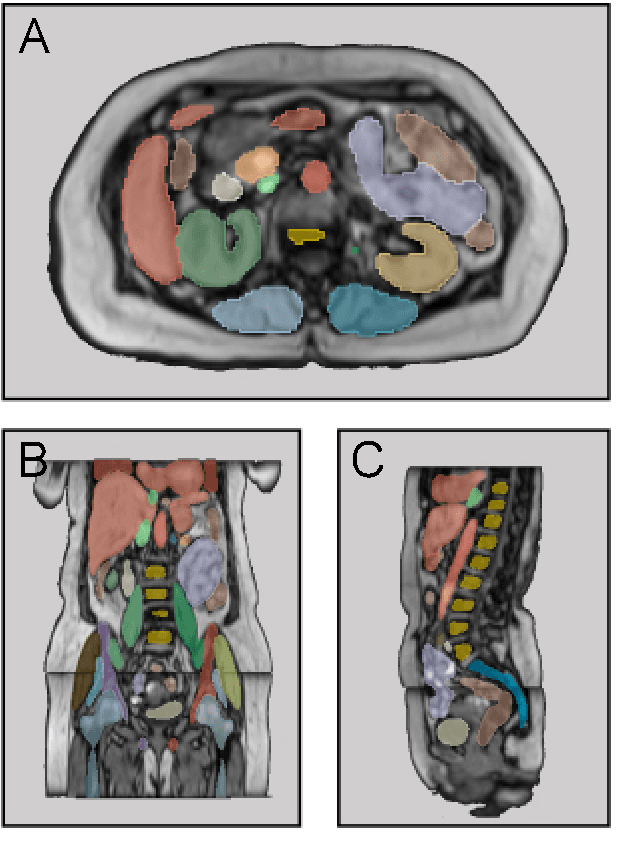
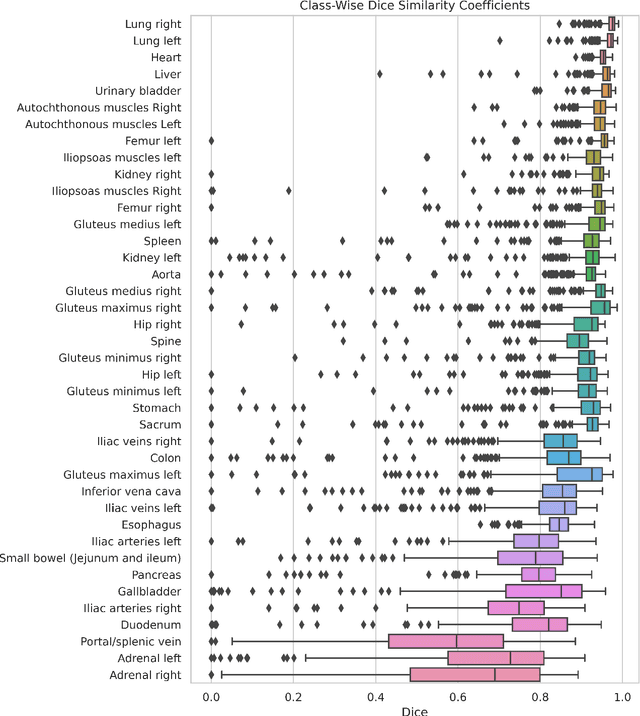
Abstract:Purpose: To introduce a deep learning model capable of multi-organ segmentation in MRI scans, offering a solution to the current limitations in MRI analysis due to challenges in resolution, standardized intensity values, and variability in sequences. Materials and Methods: he model was trained on 1,200 manually annotated MRI scans from the UK Biobank, 221 in-house MRI scans and 1228 CT scans, leveraging cross-modality transfer learning from CT segmentation models. A human-in-the-loop annotation workflow was employed to efficiently create high-quality segmentations. The model's performance was evaluated on NAKO and the AMOS22 dataset containing 600 and 60 MRI examinations. Dice Similarity Coefficient (DSC) and Hausdorff Distance (HD) was used to assess segmentation accuracy. The model will be open sourced. Results: The model showcased high accuracy in segmenting well-defined organs, achieving Dice Similarity Coefficient (DSC) scores of 0.97 for the right and left lungs, and 0.95 for the heart. It also demonstrated robustness in organs like the liver (DSC: 0.96) and kidneys (DSC: 0.95 left, 0.95 right), which present more variability. However, segmentation of smaller and complex structures such as the portal and splenic veins (DSC: 0.54) and adrenal glands (DSC: 0.65 left, 0.61 right) revealed the need for further model optimization. Conclusion: The proposed model is a robust, tool for accurate segmentation of 40 anatomical structures in MRI and CT images. By leveraging cross-modality learning and interactive annotation, the model achieves strong performance and generalizability across diverse datasets, making it a valuable resource for researchers and clinicians. It is open source and can be downloaded from https://github.com/hhaentze/MRSegmentator.
Improve Cross-Modality Segmentation by Treating MRI Images as Inverted CT Scans
May 04, 2024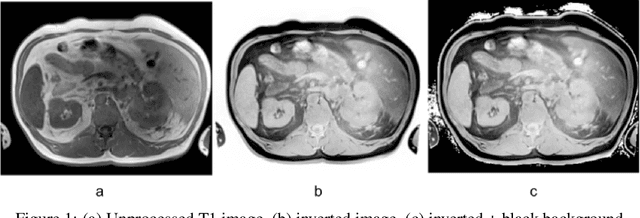
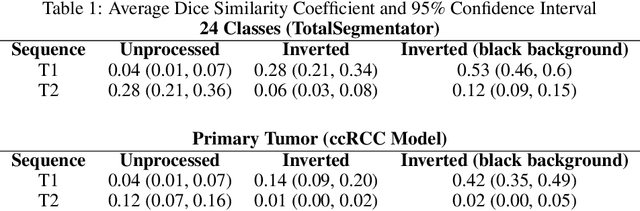
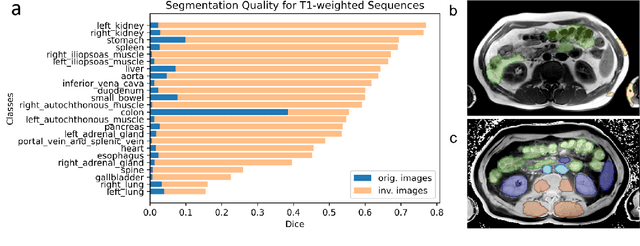
Abstract:Computed tomography (CT) segmentation models frequently include classes that are not currently supported by magnetic resonance imaging (MRI) segmentation models. In this study, we show that a simple image inversion technique can significantly improve the segmentation quality of CT segmentation models on MRI data, by using the TotalSegmentator model, applied to T1-weighted MRI images, as example. Image inversion is straightforward to implement and does not require dedicated graphics processing units (GPUs), thus providing a quick alternative to complex deep modality-transfer models for generating segmentation masks for MRI data.
Is Open-Source There Yet? A Comparative Study on Commercial and Open-Source LLMs in Their Ability to Label Chest X-Ray Reports
Feb 19, 2024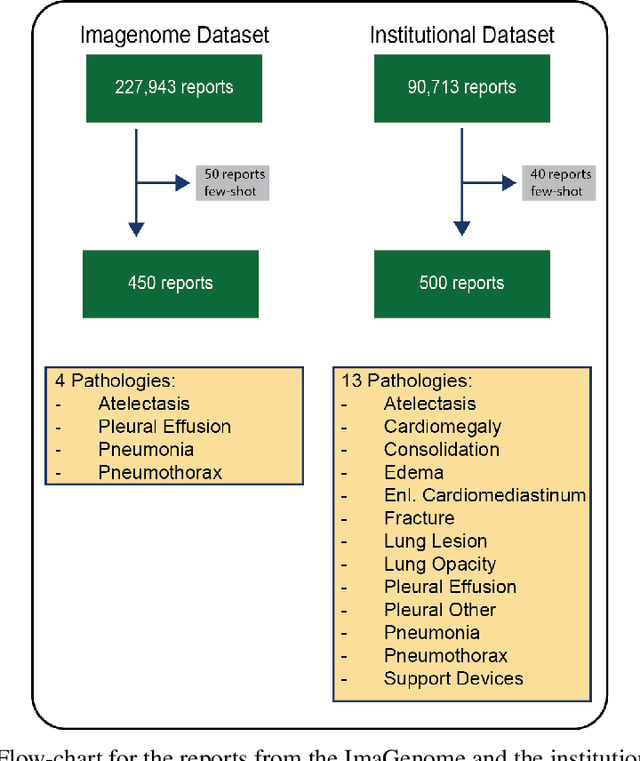
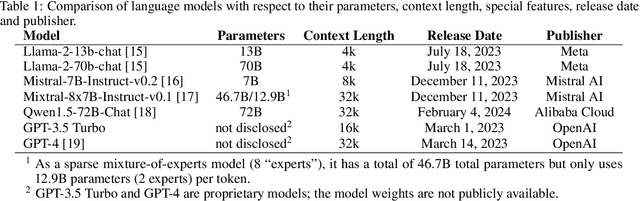
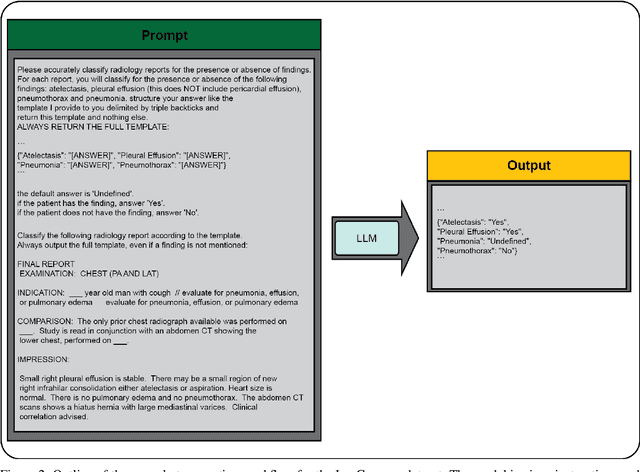
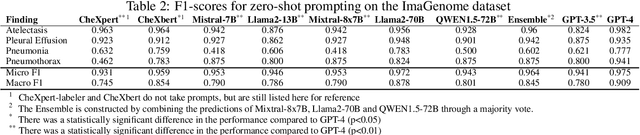
Abstract:Introduction: With the rapid advances in large language models (LLMs), there have been numerous new open source as well as commercial models. While recent publications have explored GPT-4 in its application to extracting information of interest from radiology reports, there has not been a real-world comparison of GPT-4 to different leading open-source models. Materials and Methods: Two different and independent datasets were used. The first dataset consists of 540 chest x-ray reports that were created at the Massachusetts General Hospital between July 2019 and July 2021. The second dataset consists of 500 chest x-ray reports from the ImaGenome dataset. We then compared the commercial models GPT-3.5 Turbo and GPT-4 from OpenAI to the open-source models Mistral-7B, Mixtral-8x7B, Llama2-13B, Llama2-70B, QWEN1.5-72B and CheXbert and CheXpert-labeler in their ability to accurately label the presence of multiple findings in x-ray text reports using different prompting techniques. Results: On the ImaGenome dataset, the best performing open-source model was Llama2-70B with micro F1-scores of 0.972 and 0.970 for zero- and few-shot prompts, respectively. GPT-4 achieved micro F1-scores of 0.975 and 0.984, respectively. On the institutional dataset, the best performing open-source model was QWEN1.5-72B with micro F1-scores of 0.952 and 0.965 for zero- and few-shot prompting, respectively. GPT-4 achieved micro F1-scores of 0.975 and 0.973, respectively. Conclusion: In this paper, we show that while GPT-4 is superior to open-source models in zero-shot report labeling, the implementation of few-shot prompting can bring open-source models on par with GPT-4. This shows that open-source models could be a performant and privacy preserving alternative to GPT-4 for the task of radiology report classification.
MedAlpaca -- An Open-Source Collection of Medical Conversational AI Models and Training Data
Apr 14, 2023Abstract:As large language models (LLMs) like OpenAI's GPT series continue to make strides, we witness the emergence of artificial intelligence applications in an ever-expanding range of fields. In medicine, these LLMs hold considerable promise for improving medical workflows, diagnostics, patient care, and education. Yet, there is an urgent need for open-source models that can be deployed on-premises to safeguard patient privacy. In our work, we present an innovative dataset consisting of over 160,000 entries, specifically crafted to fine-tune LLMs for effective medical applications. We investigate the impact of fine-tuning these datasets on publicly accessible pre-trained LLMs, and subsequently, we juxtapose the performance of pre-trained-only models against the fine-tuned models concerning the examinations that future medical doctors must pass to achieve certification.
MEDBERT.de: A Comprehensive German BERT Model for the Medical Domain
Mar 24, 2023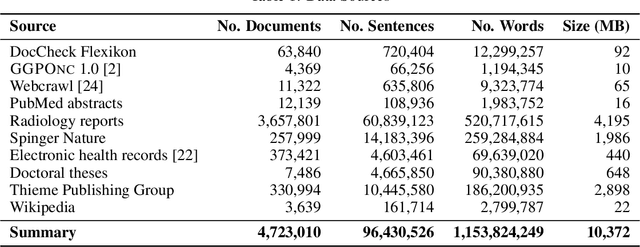
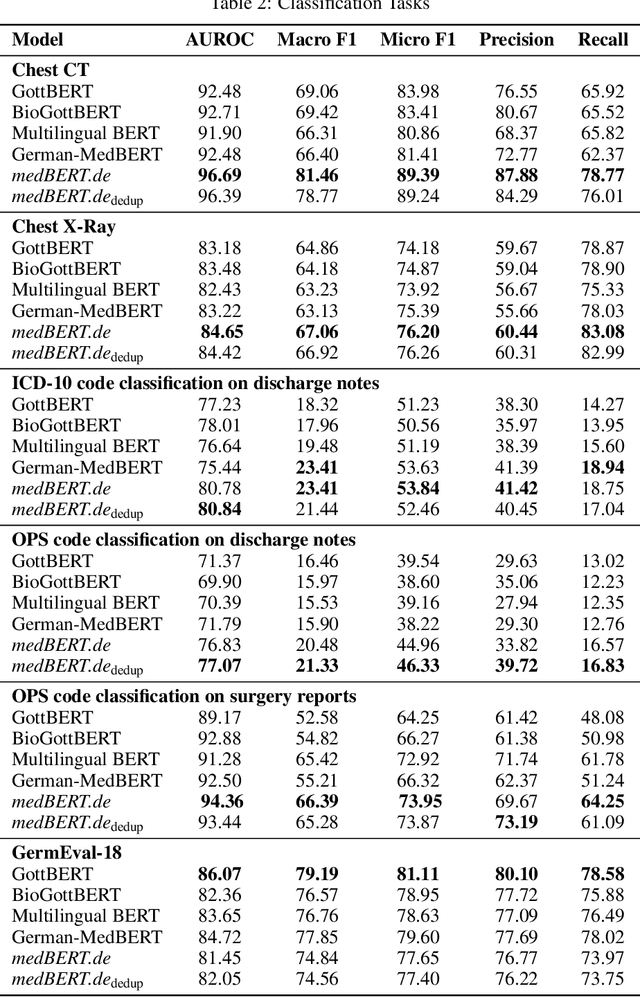
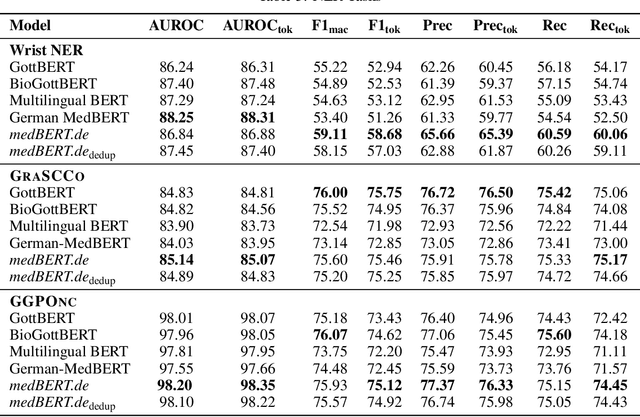
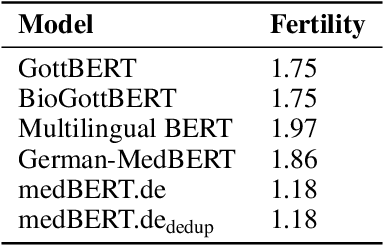
Abstract:This paper presents medBERTde, a pre-trained German BERT model specifically designed for the German medical domain. The model has been trained on a large corpus of 4.7 Million German medical documents and has been shown to achieve new state-of-the-art performance on eight different medical benchmarks covering a wide range of disciplines and medical document types. In addition to evaluating the overall performance of the model, this paper also conducts a more in-depth analysis of its capabilities. We investigate the impact of data deduplication on the model's performance, as well as the potential benefits of using more efficient tokenization methods. Our results indicate that domain-specific models such as medBERTde are particularly useful for longer texts, and that deduplication of training data does not necessarily lead to improved performance. Furthermore, we found that efficient tokenization plays only a minor role in improving model performance, and attribute most of the improved performance to the large amount of training data. To encourage further research, the pre-trained model weights and new benchmarks based on radiological data are made publicly available for use by the scientific community.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge