David G. T. Barrett
Advancing Conversational Diagnostic AI with Multimodal Reasoning
May 06, 2025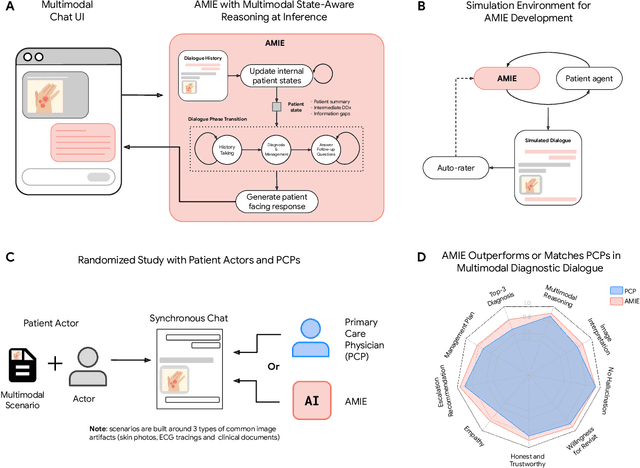
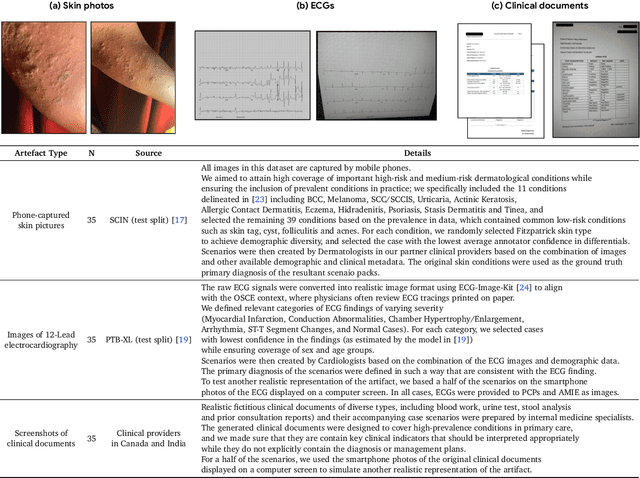
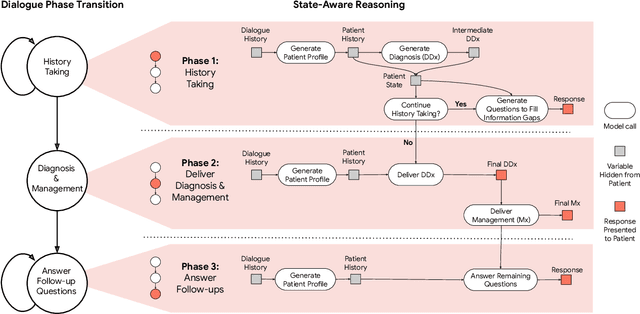
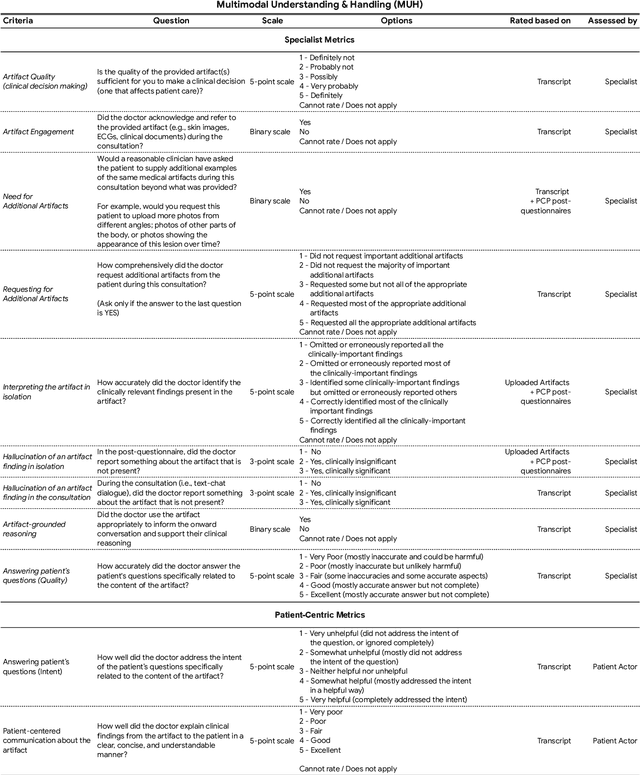
Abstract:Large Language Models (LLMs) have demonstrated great potential for conducting diagnostic conversations but evaluation has been largely limited to language-only interactions, deviating from the real-world requirements of remote care delivery. Instant messaging platforms permit clinicians and patients to upload and discuss multimodal medical artifacts seamlessly in medical consultation, but the ability of LLMs to reason over such data while preserving other attributes of competent diagnostic conversation remains unknown. Here we advance the conversational diagnosis and management performance of the Articulate Medical Intelligence Explorer (AMIE) through a new capability to gather and interpret multimodal data, and reason about this precisely during consultations. Leveraging Gemini 2.0 Flash, our system implements a state-aware dialogue framework, where conversation flow is dynamically controlled by intermediate model outputs reflecting patient states and evolving diagnoses. Follow-up questions are strategically directed by uncertainty in such patient states, leading to a more structured multimodal history-taking process that emulates experienced clinicians. We compared AMIE to primary care physicians (PCPs) in a randomized, blinded, OSCE-style study of chat-based consultations with patient actors. We constructed 105 evaluation scenarios using artifacts like smartphone skin photos, ECGs, and PDFs of clinical documents across diverse conditions and demographics. Our rubric assessed multimodal capabilities and other clinically meaningful axes like history-taking, diagnostic accuracy, management reasoning, communication, and empathy. Specialist evaluation showed AMIE to be superior to PCPs on 7/9 multimodal and 29/32 non-multimodal axes (including diagnostic accuracy). The results show clear progress in multimodal conversational diagnostic AI, but real-world translation needs further research.
Advancing Multimodal Medical Capabilities of Gemini
May 06, 2024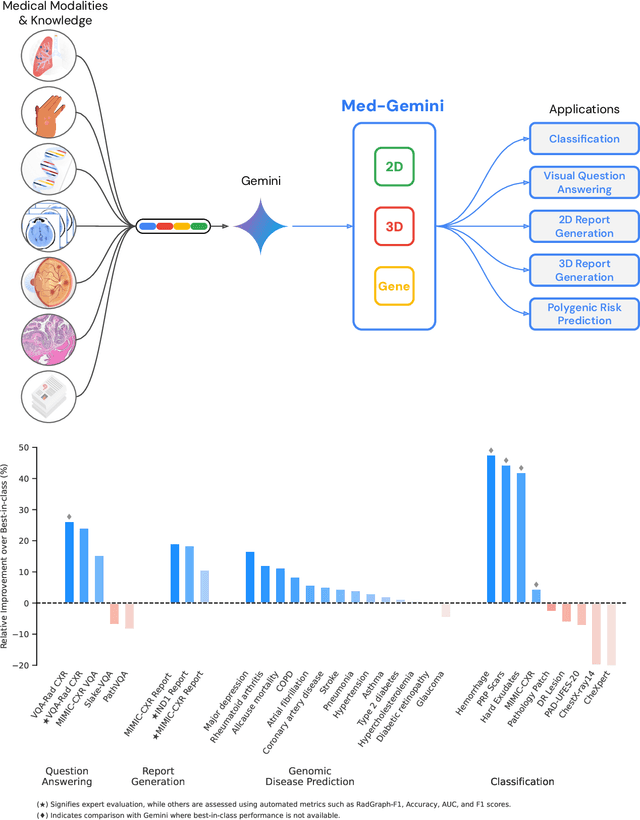
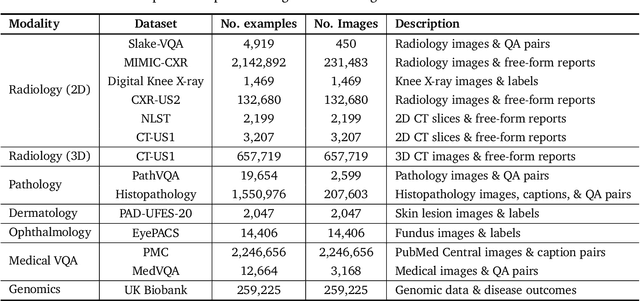

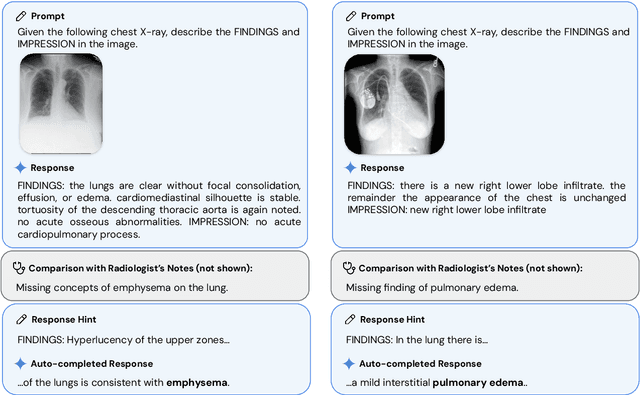
Abstract:Many clinical tasks require an understanding of specialized data, such as medical images and genomics, which is not typically found in general-purpose large multimodal models. Building upon Gemini's multimodal models, we develop several models within the new Med-Gemini family that inherit core capabilities of Gemini and are optimized for medical use via fine-tuning with 2D and 3D radiology, histopathology, ophthalmology, dermatology and genomic data. Med-Gemini-2D sets a new standard for AI-based chest X-ray (CXR) report generation based on expert evaluation, exceeding previous best results across two separate datasets by an absolute margin of 1% and 12%, where 57% and 96% of AI reports on normal cases, and 43% and 65% on abnormal cases, are evaluated as "equivalent or better" than the original radiologists' reports. We demonstrate the first ever large multimodal model-based report generation for 3D computed tomography (CT) volumes using Med-Gemini-3D, with 53% of AI reports considered clinically acceptable, although additional research is needed to meet expert radiologist reporting quality. Beyond report generation, Med-Gemini-2D surpasses the previous best performance in CXR visual question answering (VQA) and performs well in CXR classification and radiology VQA, exceeding SoTA or baselines on 17 of 20 tasks. In histopathology, ophthalmology, and dermatology image classification, Med-Gemini-2D surpasses baselines across 18 out of 20 tasks and approaches task-specific model performance. Beyond imaging, Med-Gemini-Polygenic outperforms the standard linear polygenic risk score-based approach for disease risk prediction and generalizes to genetically correlated diseases for which it has never been trained. Although further development and evaluation are necessary in the safety-critical medical domain, our results highlight the potential of Med-Gemini across a wide range of medical tasks.
Capabilities of Gemini Models in Medicine
May 01, 2024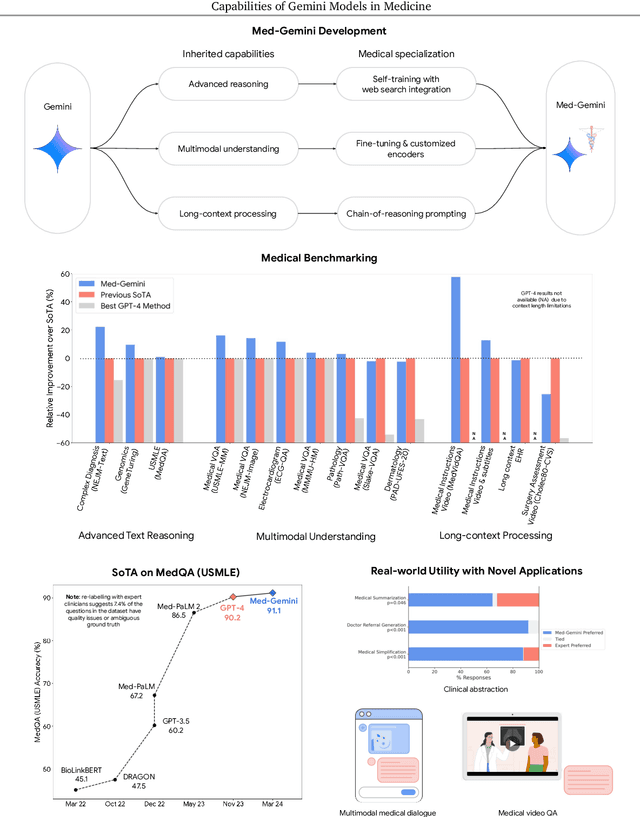
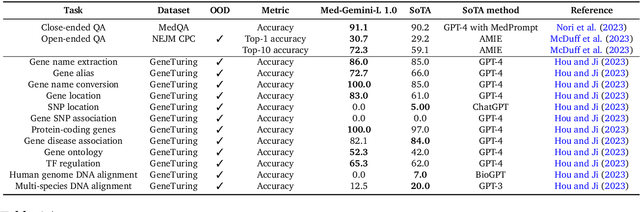
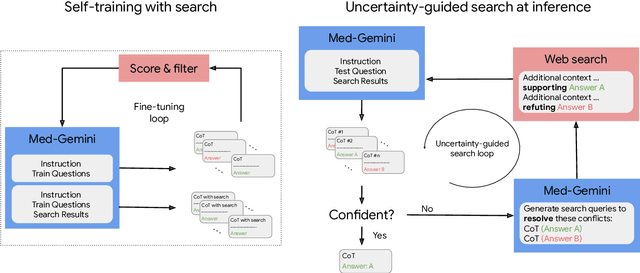
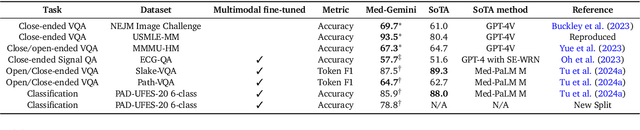
Abstract:Excellence in a wide variety of medical applications poses considerable challenges for AI, requiring advanced reasoning, access to up-to-date medical knowledge and understanding of complex multimodal data. Gemini models, with strong general capabilities in multimodal and long-context reasoning, offer exciting possibilities in medicine. Building on these core strengths of Gemini, we introduce Med-Gemini, a family of highly capable multimodal models that are specialized in medicine with the ability to seamlessly use web search, and that can be efficiently tailored to novel modalities using custom encoders. We evaluate Med-Gemini on 14 medical benchmarks, establishing new state-of-the-art (SoTA) performance on 10 of them, and surpass the GPT-4 model family on every benchmark where a direct comparison is viable, often by a wide margin. On the popular MedQA (USMLE) benchmark, our best-performing Med-Gemini model achieves SoTA performance of 91.1% accuracy, using a novel uncertainty-guided search strategy. On 7 multimodal benchmarks including NEJM Image Challenges and MMMU (health & medicine), Med-Gemini improves over GPT-4V by an average relative margin of 44.5%. We demonstrate the effectiveness of Med-Gemini's long-context capabilities through SoTA performance on a needle-in-a-haystack retrieval task from long de-identified health records and medical video question answering, surpassing prior bespoke methods using only in-context learning. Finally, Med-Gemini's performance suggests real-world utility by surpassing human experts on tasks such as medical text summarization, alongside demonstrations of promising potential for multimodal medical dialogue, medical research and education. Taken together, our results offer compelling evidence for Med-Gemini's potential, although further rigorous evaluation will be crucial before real-world deployment in this safety-critical domain.
Consensus, dissensus and synergy between clinicians and specialist foundation models in radiology report generation
Dec 06, 2023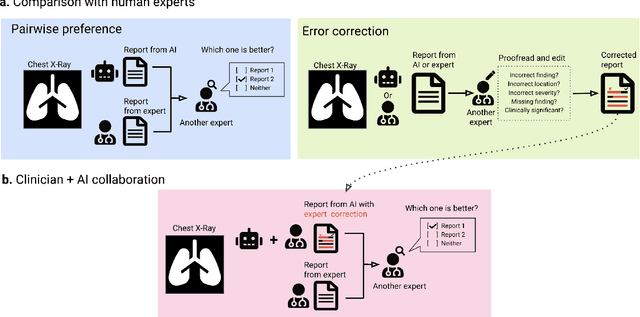
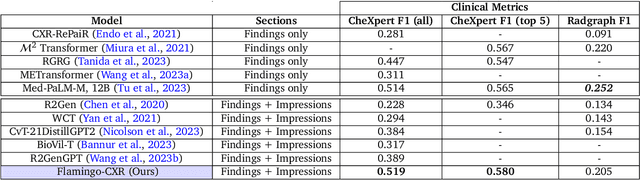
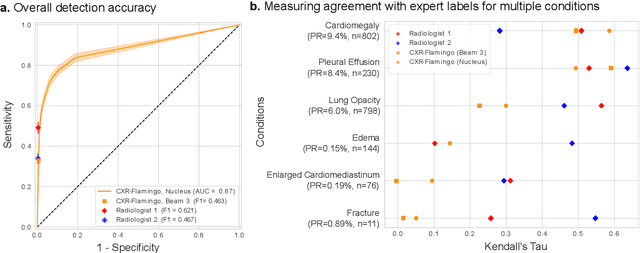
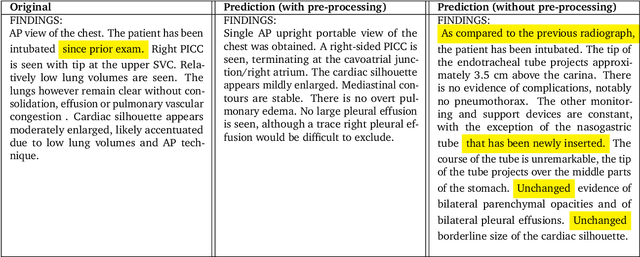
Abstract:Radiology reports are an instrumental part of modern medicine, informing key clinical decisions such as diagnosis and treatment. The worldwide shortage of radiologists, however, restricts access to expert care and imposes heavy workloads, contributing to avoidable errors and delays in report delivery. While recent progress in automated report generation with vision-language models offer clear potential in ameliorating the situation, the path to real-world adoption has been stymied by the challenge of evaluating the clinical quality of AI-generated reports. In this study, we build a state-of-the-art report generation system for chest radiographs, \textit{Flamingo-CXR}, by fine-tuning a well-known vision-language foundation model on radiology data. To evaluate the quality of the AI-generated reports, a group of 16 certified radiologists provide detailed evaluations of AI-generated and human written reports for chest X-rays from an intensive care setting in the United States and an inpatient setting in India. At least one radiologist (out of two per case) preferred the AI report to the ground truth report in over 60$\%$ of cases for both datasets. Amongst the subset of AI-generated reports that contain errors, the most frequently cited reasons were related to the location and finding, whereas for human written reports, most mistakes were related to severity and finding. This disparity suggested potential complementarity between our AI system and human experts, prompting us to develop an assistive scenario in which \textit{Flamingo-CXR} generates a first-draft report, which is subsequently revised by a clinician. This is the first demonstration of clinician-AI collaboration for report writing, and the resultant reports are assessed to be equivalent or preferred by at least one radiologist to reports written by experts alone in 80$\%$ of in-patient cases and 60$\%$ of intensive care cases.
Why neural networks find simple solutions: the many regularizers of geometric complexity
Sep 27, 2022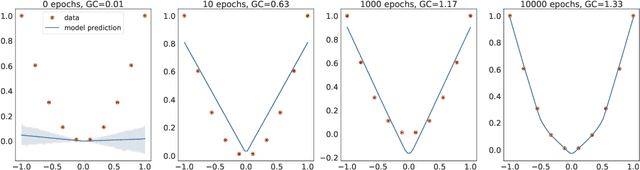
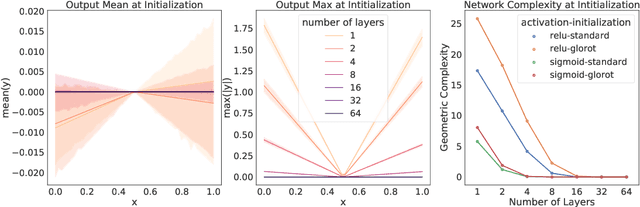
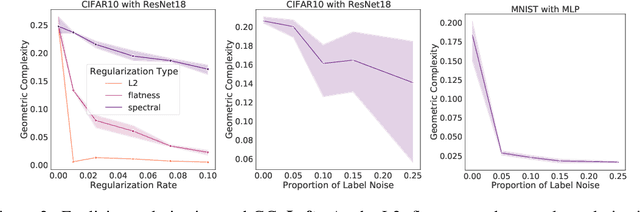
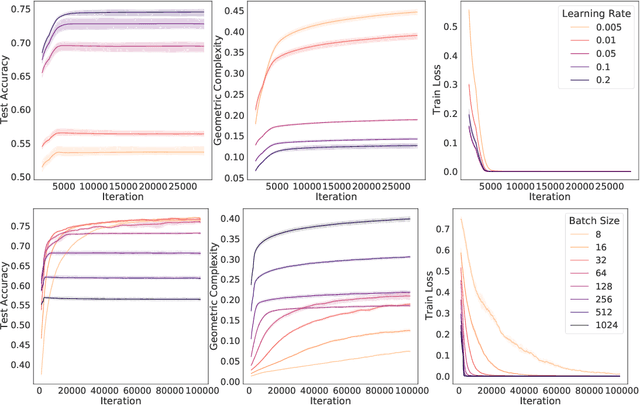
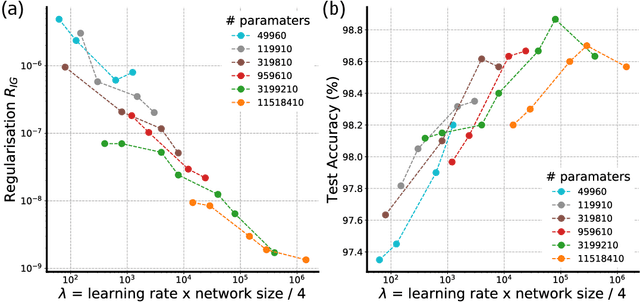
Abstract:In many contexts, simpler models are preferable to more complex models and the control of this model complexity is the goal for many methods in machine learning such as regularization, hyperparameter tuning and architecture design. In deep learning, it has been difficult to understand the underlying mechanisms of complexity control, since many traditional measures are not naturally suitable for deep neural networks. Here we develop the notion of geometric complexity, which is a measure of the variability of the model function, computed using a discrete Dirichlet energy. Using a combination of theoretical arguments and empirical results, we show that many common training heuristics such as parameter norm regularization, spectral norm regularization, flatness regularization, implicit gradient regularization, noise regularization and the choice of parameter initialization all act to control geometric complexity, providing a unifying framework in which to characterize the behavior of deep learning models.
The Geometric Occam's Razor Implicit in Deep Learning
Dec 01, 2021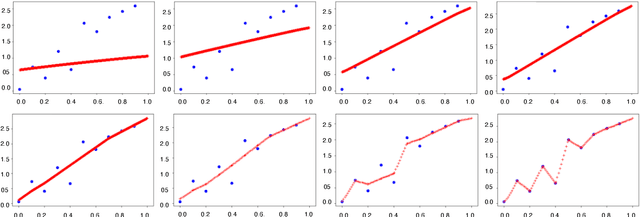
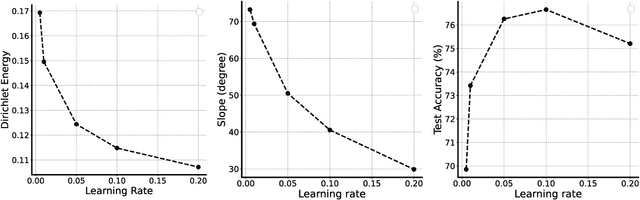
Abstract:In over-parameterized deep neural networks there can be many possible parameter configurations that fit the training data exactly. However, the properties of these interpolating solutions are poorly understood. We argue that over-parameterized neural networks trained with stochastic gradient descent are subject to a Geometric Occam's Razor; that is, these networks are implicitly regularized by the geometric model complexity. For one-dimensional regression, the geometric model complexity is simply given by the arc length of the function. For higher-dimensional settings, the geometric model complexity depends on the Dirichlet energy of the function. We explore the relationship between this Geometric Occam's Razor, the Dirichlet energy and other known forms of implicit regularization. Finally, for ResNets trained on CIFAR-10, we observe that Dirichlet energy measurements are consistent with the action of this implicit Geometric Occam's Razor.
Discretization Drift in Two-Player Games
May 28, 2021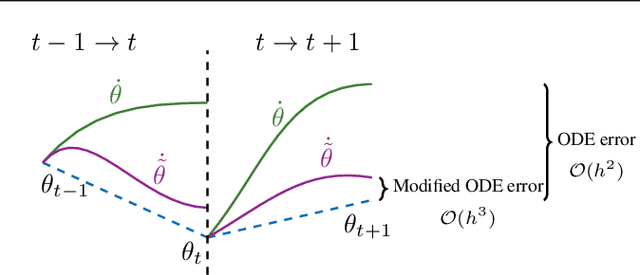
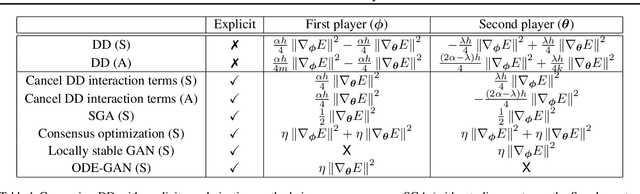
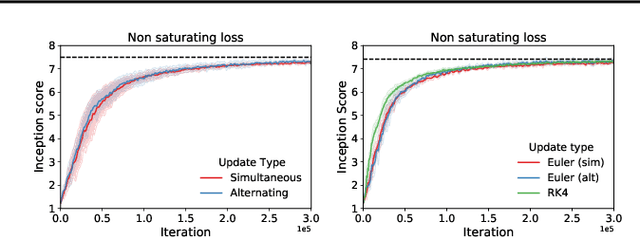
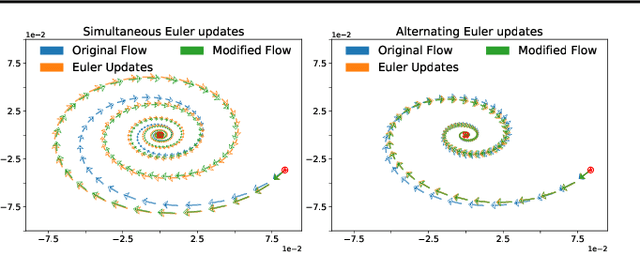
Abstract:Gradient-based methods for two-player games produce rich dynamics that can solve challenging problems, yet can be difficult to stabilize and understand. Part of this complexity originates from the discrete update steps given by simultaneous or alternating gradient descent, which causes each player to drift away from the continuous gradient flow -- a phenomenon we call discretization drift. Using backward error analysis, we derive modified continuous dynamical systems that closely follow the discrete dynamics. These modified dynamics provide an insight into the notorious challenges associated with zero-sum games, including Generative Adversarial Networks. In particular, we identify distinct components of the discretization drift that can alter performance and in some cases destabilize the game. Finally, quantifying discretization drift allows us to identify regularizers that explicitly cancel harmful forms of drift or strengthen beneficial forms of drift, and thus improve performance of GAN training.
On the Origin of Implicit Regularization in Stochastic Gradient Descent
Jan 28, 2021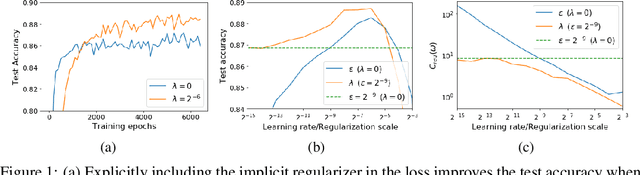
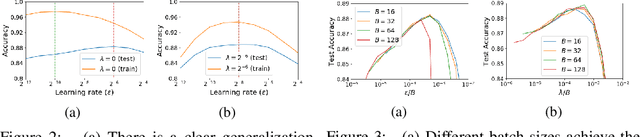
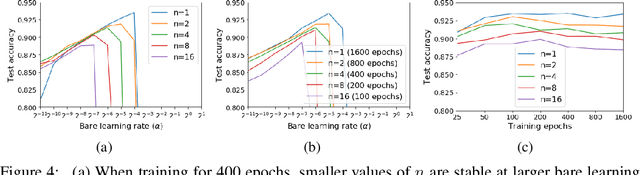
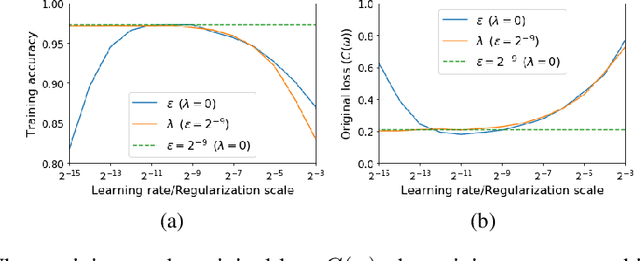
Abstract:For infinitesimal learning rates, stochastic gradient descent (SGD) follows the path of gradient flow on the full batch loss function. However moderately large learning rates can achieve higher test accuracies, and this generalization benefit is not explained by convergence bounds, since the learning rate which maximizes test accuracy is often larger than the learning rate which minimizes training loss. To interpret this phenomenon we prove that for SGD with random shuffling, the mean SGD iterate also stays close to the path of gradient flow if the learning rate is small and finite, but on a modified loss. This modified loss is composed of the original loss function and an implicit regularizer, which penalizes the norms of the minibatch gradients. Under mild assumptions, when the batch size is small the scale of the implicit regularization term is proportional to the ratio of the learning rate to the batch size. We verify empirically that explicitly including the implicit regularizer in the loss can enhance the test accuracy when the learning rate is small.
Implicit Gradient Regularization
Sep 23, 2020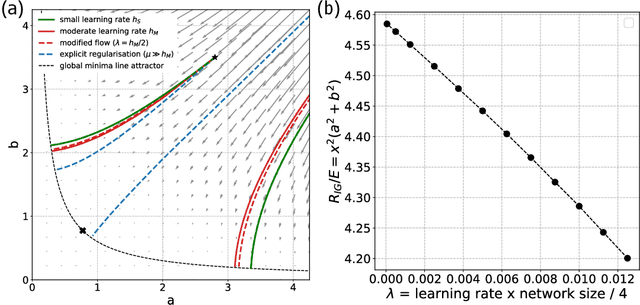
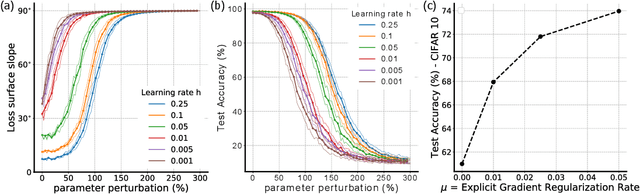
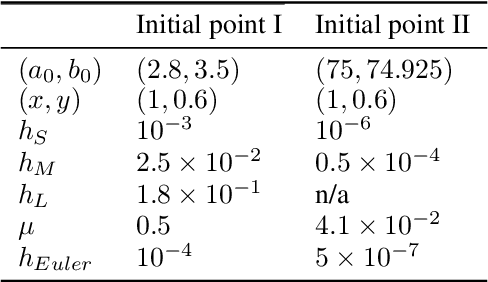
Abstract:Gradient descent can be surprisingly good at optimizing deep neural networks without overfitting and without explicit regularization. We find that the discrete steps of gradient descent implicitly regularize models by penalizing gradient descent trajectories that have large loss gradients. We call this Implicit Gradient Regularization (IGR) and we use backward error analysis to calculate the size of this regularization. We confirm empirically that implicit gradient regularization biases gradient descent toward flat minima, where test errors are small and solutions are robust to noisy parameter perturbations. Furthermore, we demonstrate that the implicit gradient regularization term can be used as an explicit regularizer, allowing us to control this gradient regularization directly. More broadly, our work indicates that backward error analysis is a useful theoretical approach to the perennial question of how learning rate, model size, and parameter regularization interact to determine the properties of overparameterized models optimized with gradient descent.
Learning to Make Analogies by Contrasting Abstract Relational Structure
Jan 31, 2019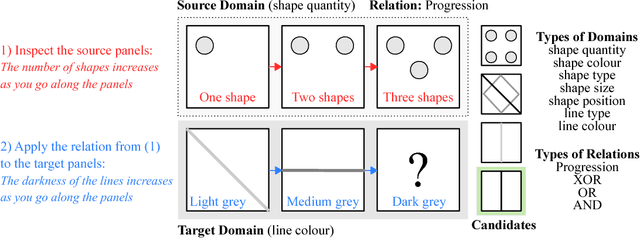
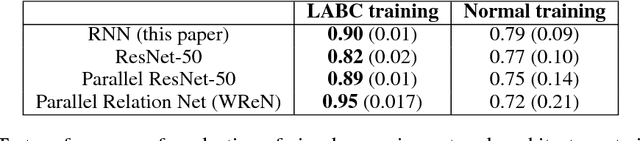
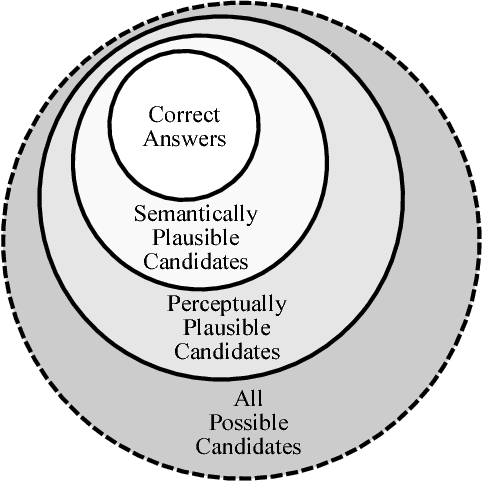
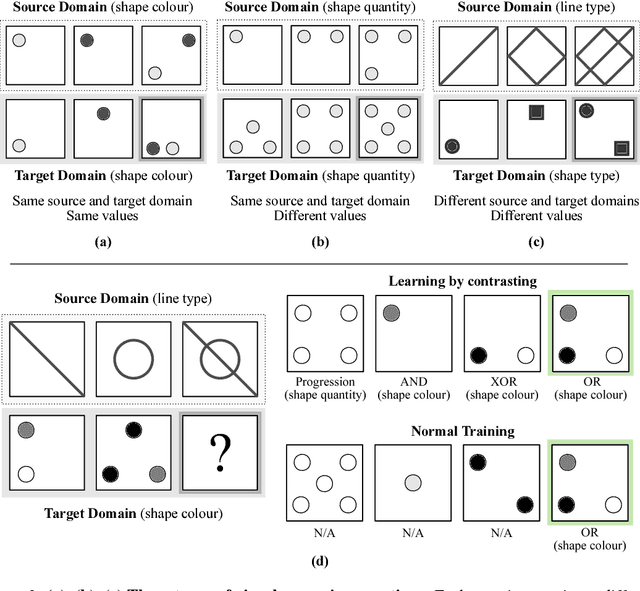
Abstract:Analogical reasoning has been a principal focus of various waves of AI research. Analogy is particularly challenging for machines because it requires relational structures to be represented such that they can be flexibly applied across diverse domains of experience. Here, we study how analogical reasoning can be induced in neural networks that learn to perceive and reason about raw visual data. We find that the critical factor for inducing such a capacity is not an elaborate architecture, but rather, careful attention to the choice of data and the manner in which it is presented to the model. The most robust capacity for analogical reasoning is induced when networks learn analogies by contrasting abstract relational structures in their input domains, a training method that uses only the input data to force models to learn about important abstract features. Using this technique we demonstrate capacities for complex, visual and symbolic analogy making and generalisation in even the simplest neural network architectures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge