Farhad Hormozdiari
Advancing Multimodal Medical Capabilities of Gemini
May 06, 2024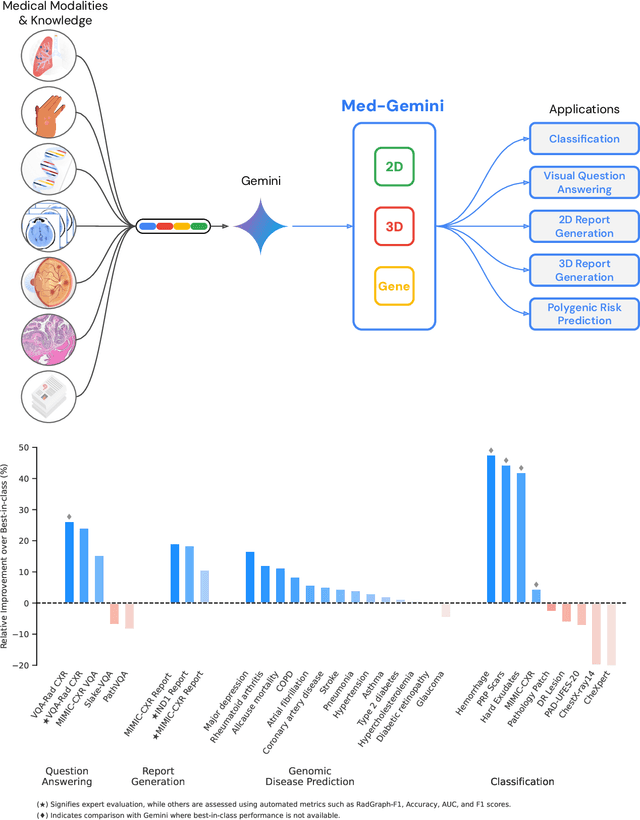
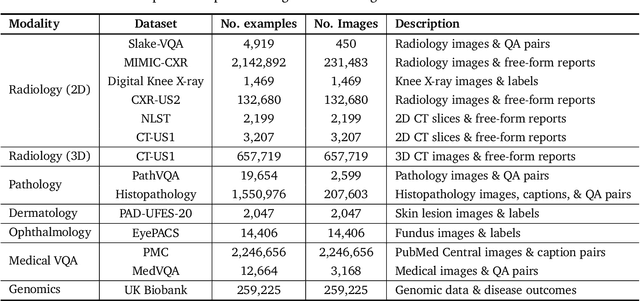

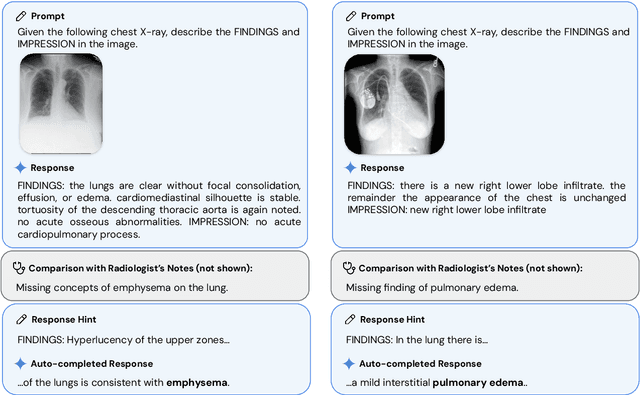
Abstract:Many clinical tasks require an understanding of specialized data, such as medical images and genomics, which is not typically found in general-purpose large multimodal models. Building upon Gemini's multimodal models, we develop several models within the new Med-Gemini family that inherit core capabilities of Gemini and are optimized for medical use via fine-tuning with 2D and 3D radiology, histopathology, ophthalmology, dermatology and genomic data. Med-Gemini-2D sets a new standard for AI-based chest X-ray (CXR) report generation based on expert evaluation, exceeding previous best results across two separate datasets by an absolute margin of 1% and 12%, where 57% and 96% of AI reports on normal cases, and 43% and 65% on abnormal cases, are evaluated as "equivalent or better" than the original radiologists' reports. We demonstrate the first ever large multimodal model-based report generation for 3D computed tomography (CT) volumes using Med-Gemini-3D, with 53% of AI reports considered clinically acceptable, although additional research is needed to meet expert radiologist reporting quality. Beyond report generation, Med-Gemini-2D surpasses the previous best performance in CXR visual question answering (VQA) and performs well in CXR classification and radiology VQA, exceeding SoTA or baselines on 17 of 20 tasks. In histopathology, ophthalmology, and dermatology image classification, Med-Gemini-2D surpasses baselines across 18 out of 20 tasks and approaches task-specific model performance. Beyond imaging, Med-Gemini-Polygenic outperforms the standard linear polygenic risk score-based approach for disease risk prediction and generalizes to genetically correlated diseases for which it has never been trained. Although further development and evaluation are necessary in the safety-critical medical domain, our results highlight the potential of Med-Gemini across a wide range of medical tasks.
Multimodal LLMs for health grounded in individual-specific data
Jul 20, 2023



Abstract:Foundation large language models (LLMs) have shown an impressive ability to solve tasks across a wide range of fields including health. To effectively solve personalized health tasks, LLMs need the ability to ingest a diversity of data modalities that are relevant to an individual's health status. In this paper, we take a step towards creating multimodal LLMs for health that are grounded in individual-specific data by developing a framework (HeLM: Health Large Language Model for Multimodal Understanding) that enables LLMs to use high-dimensional clinical modalities to estimate underlying disease risk. HeLM encodes complex data modalities by learning an encoder that maps them into the LLM's token embedding space and for simple modalities like tabular data by serializing the data into text. Using data from the UK Biobank, we show that HeLM can effectively use demographic and clinical features in addition to high-dimensional time-series data to estimate disease risk. For example, HeLM achieves an AUROC of 0.75 for asthma prediction when combining tabular and spirogram data modalities compared with 0.49 when only using tabular data. Overall, we find that HeLM outperforms or performs at parity with classical machine learning approaches across a selection of eight binary traits. Furthermore, we investigate the downstream uses of this model such as its generalizability to out-of-distribution traits and its ability to power conversations around individual health and wellness.
Underspecification Presents Challenges for Credibility in Modern Machine Learning
Nov 06, 2020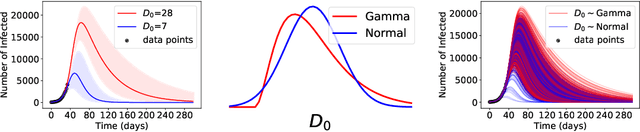


Abstract:ML models often exhibit unexpectedly poor behavior when they are deployed in real-world domains. We identify underspecification as a key reason for these failures. An ML pipeline is underspecified when it can return many predictors with equivalently strong held-out performance in the training domain. Underspecification is common in modern ML pipelines, such as those based on deep learning. Predictors returned by underspecified pipelines are often treated as equivalent based on their training domain performance, but we show here that such predictors can behave very differently in deployment domains. This ambiguity can lead to instability and poor model behavior in practice, and is a distinct failure mode from previously identified issues arising from structural mismatch between training and deployment domains. We show that this problem appears in a wide variety of practical ML pipelines, using examples from computer vision, medical imaging, natural language processing, clinical risk prediction based on electronic health records, and medical genomics. Our results show the need to explicitly account for underspecification in modeling pipelines that are intended for real-world deployment in any domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge