Daniel Shu Wei Ting
Toward Global Large Language Models in Medicine
Jan 05, 2026Abstract:Despite continuous advances in medical technology, the global distribution of health care resources remains uneven. The development of large language models (LLMs) has transformed the landscape of medicine and holds promise for improving health care quality and expanding access to medical information globally. However, existing LLMs are primarily trained on high-resource languages, limiting their applicability in global medical scenarios. To address this gap, we constructed GlobMed, a large multilingual medical dataset, containing over 500,000 entries spanning 12 languages, including four low-resource languages. Building on this, we established GlobMed-Bench, which systematically assesses 56 state-of-the-art proprietary and open-weight LLMs across multiple multilingual medical tasks, revealing significant performance disparities across languages, particularly for low-resource languages. Additionally, we introduced GlobMed-LLMs, a suite of multilingual medical LLMs trained on GlobMed, with parameters ranging from 1.7B to 8B. GlobMed-LLMs achieved an average performance improvement of over 40% relative to baseline models, with a more than threefold increase in performance on low-resource languages. Together, these resources provide an important foundation for advancing the equitable development and application of LLMs globally, enabling broader language communities to benefit from technological advances.
The Evolving Landscape of Generative Large Language Models and Traditional Natural Language Processing in Medicine
May 15, 2025Abstract:Natural language processing (NLP) has been traditionally applied to medicine, and generative large language models (LLMs) have become prominent recently. However, the differences between them across different medical tasks remain underexplored. We analyzed 19,123 studies, finding that generative LLMs demonstrate advantages in open-ended tasks, while traditional NLP dominates in information extraction and analysis tasks. As these technologies advance, ethical use of them is essential to ensure their potential in medical applications.
Real-world Deployment and Evaluation of PErioperative AI CHatbot (PEACH) -- a Large Language Model Chatbot for Perioperative Medicine
Dec 24, 2024



Abstract:Large Language Models (LLMs) are emerging as powerful tools in healthcare, particularly for complex, domain-specific tasks. This study describes the development and evaluation of the PErioperative AI CHatbot (PEACH), a secure LLM-based system integrated with local perioperative guidelines to support preoperative clinical decision-making. PEACH was embedded with 35 institutional perioperative protocols in the secure Claude 3.5 Sonet LLM framework within Pair Chat (developed by Singapore Government) and tested in a silent deployment with real-world data. Accuracy, safety, and usability were assessed. Deviations and hallucinations were categorized based on potential harm, and user feedback was evaluated using the Technology Acceptance Model (TAM). Updates were made after the initial silent deployment to amend one protocol. In 240 real-world clinical iterations, PEACH achieved a first-generation accuracy of 97.5% (78/80) and an overall accuracy of 96.7% (232/240) across three iterations. The updated PEACH demonstrated improved accuracy of 97.9% (235/240), with a statistically significant difference from the null hypothesis of 95% accuracy (p = 0.018, 95% CI: 0.952-0.991). Minimal hallucinations and deviations were observed (both 1/240 and 2/240, respectively). Clinicians reported that PEACH expedited decisions in 95% of cases, and inter-rater reliability ranged from kappa 0.772-0.893 within PEACH and 0.610-0.784 among attendings. PEACH is an accurate, adaptable tool that enhances consistency and efficiency in perioperative decision-making. Future research should explore its scalability across specialties and its impact on clinical outcomes.
oRetrieval Augmented Generation for 10 Large Language Models and its Generalizability in Assessing Medical Fitness
Oct 11, 2024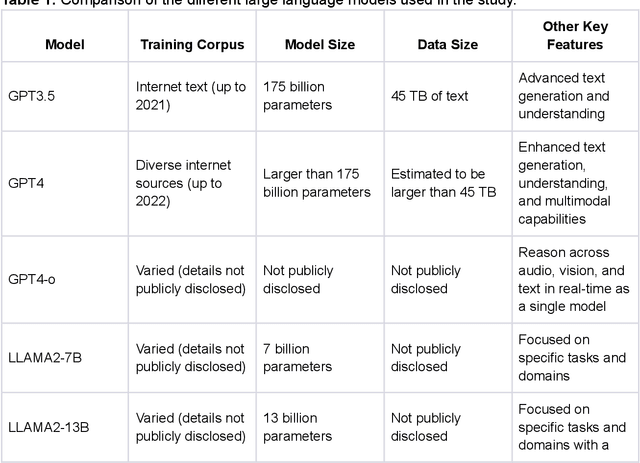



Abstract:Large Language Models (LLMs) show potential for medical applications but often lack specialized clinical knowledge. Retrieval Augmented Generation (RAG) allows customization with domain-specific information, making it suitable for healthcare. This study evaluates the accuracy, consistency, and safety of RAG models in determining fitness for surgery and providing preoperative instructions. We developed LLM-RAG models using 35 local and 23 international preoperative guidelines and tested them against human-generated responses. A total of 3,682 responses were evaluated. Clinical documents were processed using Llamaindex, and 10 LLMs, including GPT3.5, GPT4, and Claude-3, were assessed. Fourteen clinical scenarios were analyzed, focusing on seven aspects of preoperative instructions. Established guidelines and expert judgment were used to determine correct responses, with human-generated answers serving as comparisons. The LLM-RAG models generated responses within 20 seconds, significantly faster than clinicians (10 minutes). The GPT4 LLM-RAG model achieved the highest accuracy (96.4% vs. 86.6%, p=0.016), with no hallucinations and producing correct instructions comparable to clinicians. Results were consistent across both local and international guidelines. This study demonstrates the potential of LLM-RAG models for preoperative healthcare tasks, highlighting their efficiency, scalability, and reliability.
From Generalist to Specialist: Adapting Vision Language Models via Task-Specific Visual Instruction Tuning
Oct 09, 2024



Abstract:Large vision language models (VLMs) combine large language models with vision encoders, demonstrating promise across various tasks. However, they often underperform in task-specific applications due to domain gaps between pre-training and fine-tuning. We introduce VITask, a novel framework that enhances task-specific adaptability of VLMs by integrating task-specific models (TSMs). VITask employs three key strategies: exemplar prompting (EP), response distribution alignment (RDA), and contrastive response tuning (CRT) to improve the task-specific performance of VLMs by adjusting their response distributions. EP allows TSM features to guide VLMs, while RDA enables VLMs to adapt without TSMs during inference by learning from exemplar-prompted models. CRT further optimizes the ranking of correct image-response pairs, thereby reducing the risk of generating undesired responses. Experiments on 12 medical diagnosis datasets across 9 imaging modalities show that VITask outperforms both vanilla instruction-tuned VLMs and TSMs, showcasing its ability to integrate complementary features from both models effectively. Additionally, VITask offers practical advantages such as flexible TSM integration and robustness to incomplete instructions, making it a versatile and efficient solution for task-specific VLM tuning. Our code are available at https://github.com/baiyang4/VITask.
Retrieval-Augmented Generation for Generative Artificial Intelligence in Medicine
Jun 18, 2024Abstract:Generative artificial intelligence (AI) has brought revolutionary innovations in various fields, including medicine. However, it also exhibits limitations. In response, retrieval-augmented generation (RAG) provides a potential solution, enabling models to generate more accurate contents by leveraging the retrieval of external knowledge. With the rapid advancement of generative AI, RAG can pave the way for connecting this transformative technology with medical applications and is expected to bring innovations in equity, reliability, and personalization to health care.
Towards Clinical AI Fairness: Filling Gaps in the Puzzle
May 28, 2024



Abstract:The ethical integration of Artificial Intelligence (AI) in healthcare necessitates addressing fairness-a concept that is highly context-specific across medical fields. Extensive studies have been conducted to expand the technical components of AI fairness, while tremendous calls for AI fairness have been raised from healthcare. Despite this, a significant disconnect persists between technical advancements and their practical clinical applications, resulting in a lack of contextualized discussion of AI fairness in clinical settings. Through a detailed evidence gap analysis, our review systematically pinpoints several deficiencies concerning both healthcare data and the provided AI fairness solutions. We highlight the scarcity of research on AI fairness in many medical domains where AI technology is increasingly utilized. Additionally, our analysis highlights a substantial reliance on group fairness, aiming to ensure equality among demographic groups from a macro healthcare system perspective; in contrast, individual fairness, focusing on equity at a more granular level, is frequently overlooked. To bridge these gaps, our review advances actionable strategies for both the healthcare and AI research communities. Beyond applying existing AI fairness methods in healthcare, we further emphasize the importance of involving healthcare professionals to refine AI fairness concepts and methods to ensure contextually relevant and ethically sound AI applications in healthcare.
Fine-tuning Large Language Model (LLM) Artificial Intelligence Chatbots in Ophthalmology and LLM-based evaluation using GPT-4
Feb 15, 2024



Abstract:Purpose: To assess the alignment of GPT-4-based evaluation to human clinician experts, for the evaluation of responses to ophthalmology-related patient queries generated by fine-tuned LLM chatbots. Methods: 400 ophthalmology questions and paired answers were created by ophthalmologists to represent commonly asked patient questions, divided into fine-tuning (368; 92%), and testing (40; 8%). We find-tuned 5 different LLMs, including LLAMA2-7b, LLAMA2-7b-Chat, LLAMA2-13b, and LLAMA2-13b-Chat. For the testing dataset, additional 8 glaucoma QnA pairs were included. 200 responses to the testing dataset were generated by 5 fine-tuned LLMs for evaluation. A customized clinical evaluation rubric was used to guide GPT-4 evaluation, grounded on clinical accuracy, relevance, patient safety, and ease of understanding. GPT-4 evaluation was then compared against ranking by 5 clinicians for clinical alignment. Results: Among all fine-tuned LLMs, GPT-3.5 scored the highest (87.1%), followed by LLAMA2-13b (80.9%), LLAMA2-13b-chat (75.5%), LLAMA2-7b-Chat (70%) and LLAMA2-7b (68.8%) based on the GPT-4 evaluation. GPT-4 evaluation demonstrated significant agreement with human clinician rankings, with Spearman and Kendall Tau correlation coefficients of 0.90 and 0.80 respectively; while correlation based on Cohen Kappa was more modest at 0.50. Notably, qualitative analysis and the glaucoma sub-analysis revealed clinical inaccuracies in the LLM-generated responses, which were appropriately identified by the GPT-4 evaluation. Conclusion: The notable clinical alignment of GPT-4 evaluation highlighted its potential to streamline the clinical evaluation of LLM chatbot responses to healthcare-related queries. By complementing the existing clinician-dependent manual grading, this efficient and automated evaluation could assist the validation of future developments in LLM applications for healthcare.
Development and Testing of Retrieval Augmented Generation in Large Language Models -- A Case Study Report
Jan 29, 2024Abstract:Purpose: Large Language Models (LLMs) hold significant promise for medical applications. Retrieval Augmented Generation (RAG) emerges as a promising approach for customizing domain knowledge in LLMs. This case study presents the development and evaluation of an LLM-RAG pipeline tailored for healthcare, focusing specifically on preoperative medicine. Methods: We developed an LLM-RAG model using 35 preoperative guidelines and tested it against human-generated responses, with a total of 1260 responses evaluated. The RAG process involved converting clinical documents into text using Python-based frameworks like LangChain and Llamaindex, and processing these texts into chunks for embedding and retrieval. Vector storage techniques and selected embedding models to optimize data retrieval, using Pinecone for vector storage with a dimensionality of 1536 and cosine similarity for loss metrics. Human-generated answers, provided by junior doctors, were used as a comparison. Results: The LLM-RAG model generated answers within an average of 15-20 seconds, significantly faster than the 10 minutes typically required by humans. Among the basic LLMs, GPT4.0 exhibited the best accuracy of 80.1%. This accuracy was further increased to 91.4% when the model was enhanced with RAG. Compared to the human-generated instructions, which had an accuracy of 86.3%, the performance of the GPT4.0 RAG model demonstrated non-inferiority (p=0.610). Conclusions: In this case study, we demonstrated a LLM-RAG model for healthcare implementation. The pipeline shows the advantages of grounded knowledge, upgradability, and scalability as important aspects of healthcare LLM deployment.
Development and Testing of a Novel Large Language Model-Based Clinical Decision Support Systems for Medication Safety in 12 Clinical Specialties
Jan 29, 2024



Abstract:Importance: We introduce a novel Retrieval Augmented Generation (RAG)-Large Language Model (LLM) as a Clinical Decision Support System (CDSS) for safe medication prescription. This model addresses the limitations of traditional rule-based CDSS by providing relevant prescribing error alerts tailored to patient context and institutional guidelines. Objective: The study evaluates the efficacy of an LLM-based CDSS in identifying medication errors across various medical and surgical case vignettes, compared to a human expert panel. It also examines clinician preferences among different CDSS integration modalities: junior pharmacist, LLM-based CDSS alone, and a combination of both. Design, Setting, and Participants: Utilizing a RAG model with GPT-4.0, the study involved 61 prescribing error scenarios within 23 clinical vignettes across 12 specialties. An expert panel assessed these cases using the PCNE classification and NCC MERP index. Three junior pharmacists independently reviewed each vignette under simulated conditions. Main Outcomes and Measures: The study assesses the LLM-based CDSS's accuracy, precision, recall, and F1 scores in identifying Drug-Related Problems (DRPs), compared to junior pharmacists alone or in an assistive mode with the CDSS. Results: The co-pilot mode of RAG-LLM significantly improved DRP identification accuracy by 22% over solo pharmacists. It showed higher recall and F1 scores, indicating better detection of severe DRPs, despite a slight decrease in precision. Accuracy varied across categories when pharmacists had access to RAG-LLM responses. Conclusions: The RAG-LLM based CDSS enhances medication error identification accuracy when used with junior pharmacists, especially in detecting severe DRPs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge