Christina Bukas
MultiOrg: A Multi-rater Organoid-detection Dataset
Oct 18, 2024Abstract:High-throughput image analysis in the biomedical domain has gained significant attention in recent years, driving advancements in drug discovery, disease prediction, and personalized medicine. Organoids, specifically, are an active area of research, providing excellent models for human organs and their functions. Automating the quantification of organoids in microscopy images would provide an effective solution to overcome substantial manual quantification bottlenecks, particularly in high-throughput image analysis. However, there is a notable lack of open biomedical datasets, in contrast to other domains, such as autonomous driving, and, notably, only few of them have attempted to quantify annotation uncertainty. In this work, we present MultiOrg a comprehensive organoid dataset tailored for object detection tasks with uncertainty quantification. This dataset comprises over 400 high-resolution 2d microscopy images and curated annotations of more than 60,000 organoids. Most importantly, it includes three label sets for the test data, independently annotated by two experts at distinct time points. We additionally provide a benchmark for organoid detection, and make the best model available through an easily installable, interactive plugin for the popular image visualization tool Napari, to perform organoid quantification.
The Brain Tumor Segmentation Challenge 2023: Glioma Segmentation in Sub-Saharan Africa Patient Population
May 30, 2023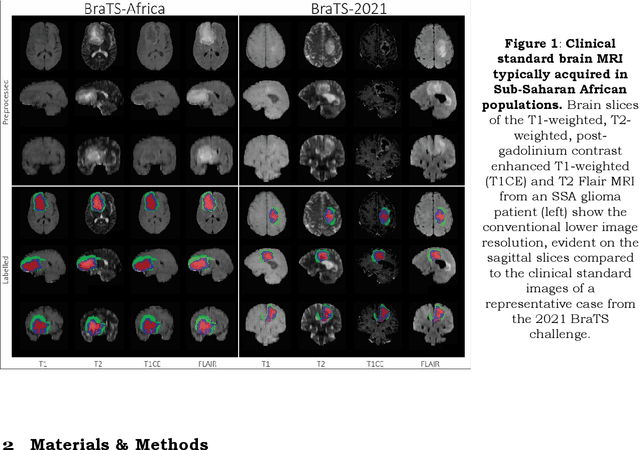
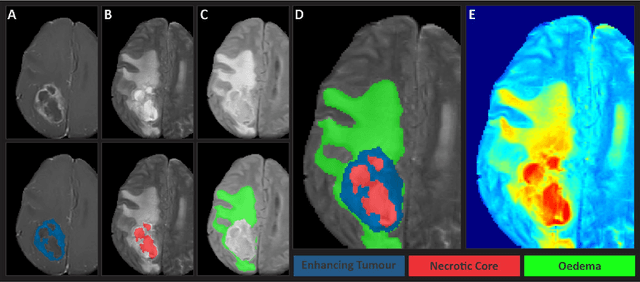
Abstract:Gliomas are the most common type of primary brain tumors. Although gliomas are relatively rare, they are among the deadliest types of cancer, with a survival rate of less than 2 years after diagnosis. Gliomas are challenging to diagnose, hard to treat and inherently resistant to conventional therapy. Years of extensive research to improve diagnosis and treatment of gliomas have decreased mortality rates across the Global North, while chances of survival among individuals in low- and middle-income countries (LMICs) remain unchanged and are significantly worse in Sub-Saharan Africa (SSA) populations. Long-term survival with glioma is associated with the identification of appropriate pathological features on brain MRI and confirmation by histopathology. Since 2012, the Brain Tumor Segmentation (BraTS) Challenge have evaluated state-of-the-art machine learning methods to detect, characterize, and classify gliomas. However, it is unclear if the state-of-the-art methods can be widely implemented in SSA given the extensive use of lower-quality MRI technology, which produces poor image contrast and resolution and more importantly, the propensity for late presentation of disease at advanced stages as well as the unique characteristics of gliomas in SSA (i.e., suspected higher rates of gliomatosis cerebri). Thus, the BraTS-Africa Challenge provides a unique opportunity to include brain MRI glioma cases from SSA in global efforts through the BraTS Challenge to develop and evaluate computer-aided-diagnostic (CAD) methods for the detection and characterization of glioma in resource-limited settings, where the potential for CAD tools to transform healthcare are more likely.
The Brain Tumor Segmentation Challenge 2023: Local Synthesis of Healthy Brain Tissue via Inpainting
May 15, 2023



Abstract:A myriad of algorithms for the automatic analysis of brain MR images is available to support clinicians in their decision-making. For brain tumor patients, the image acquisition time series typically starts with a scan that is already pathological. This poses problems, as many algorithms are designed to analyze healthy brains and provide no guarantees for images featuring lesions. Examples include but are not limited to algorithms for brain anatomy parcellation, tissue segmentation, and brain extraction. To solve this dilemma, we introduce the BraTS 2023 inpainting challenge. Here, the participants' task is to explore inpainting techniques to synthesize healthy brain scans from lesioned ones. The following manuscript contains the task formulation, dataset, and submission procedure. Later it will be updated to summarize the findings of the challenge. The challenge is organized as part of the BraTS 2023 challenge hosted at the MICCAI 2023 conference in Vancouver, Canada.
Approaching Peak Ground Truth
Dec 31, 2022

Abstract:Machine learning models are typically evaluated by computing similarity with reference annotations and trained by maximizing similarity with such. Especially in the bio-medical domain, annotations are subjective and suffer from low inter- and intra-rater reliability. Since annotations only reflect the annotation entity's interpretation of the real world, this can lead to sub-optimal predictions even though the model achieves high similarity scores. Here, the theoretical concept of Peak Ground Truth (PGT) is introduced. PGT marks the point beyond which an increase in similarity with the reference annotation stops translating to better Real World Model Performance (RWMP). Additionally, a quantitative technique to approximate PGT by computing inter- and intra-rater reliability is proposed. Finally, three categories of PGT-aware strategies to evaluate and improve model performance are reviewed.
Weakly-supervised Biomechanically-constrained CT/MRI Registration of the Spine
May 16, 2022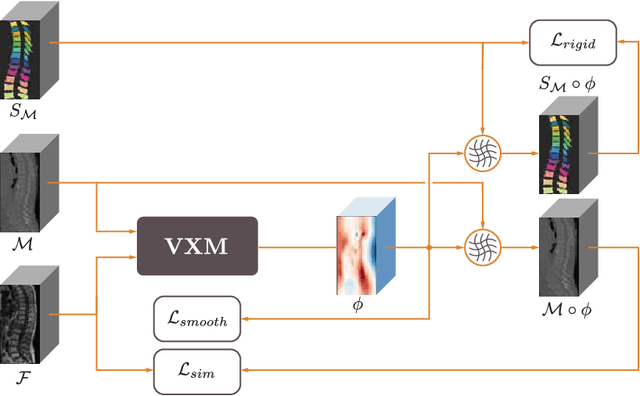
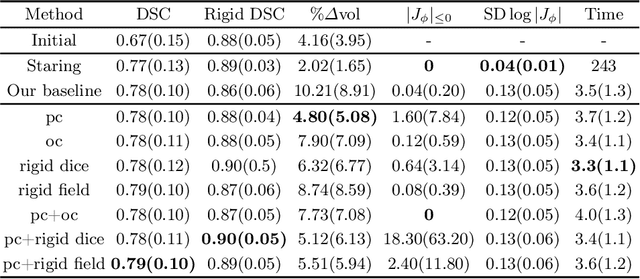
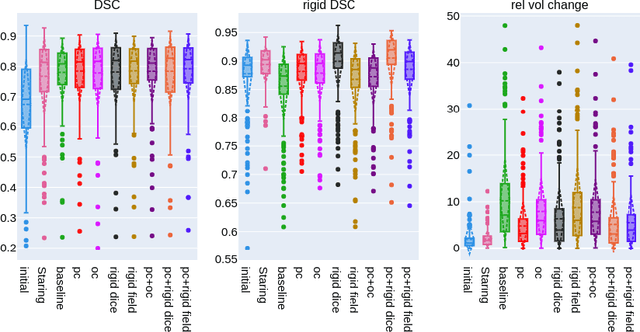
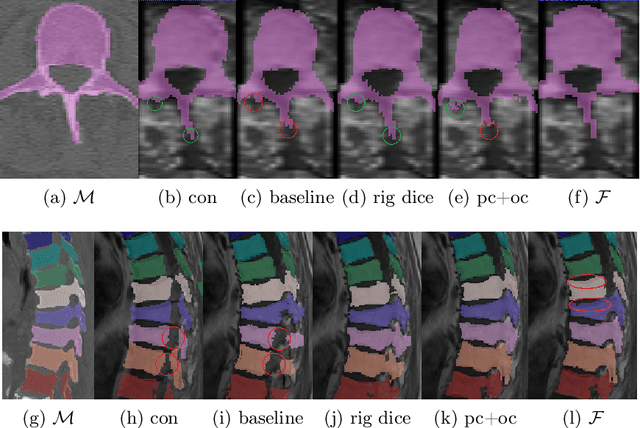
Abstract:CT and MRI are two of the most informative modalities in spinal diagnostics and treatment planning. CT is useful when analysing bony structures, while MRI gives information about the soft tissue. Thus, fusing the information of both modalities can be very beneficial. Registration is the first step for this fusion. While the soft tissues around the vertebra are deformable, each vertebral body is constrained to move rigidly. We propose a weakly-supervised deep learning framework that preserves the rigidity and the volume of each vertebra while maximizing the accuracy of the registration. To achieve this goal, we introduce anatomy-aware losses for training the network. We specifically design these losses to depend only on the CT label maps since automatic vertebra segmentation in CT gives more accurate results contrary to MRI. We evaluate our method on an in-house dataset of 167 patients. Our results show that adding the anatomy-aware losses increases the plausibility of the inferred transformation while keeping the accuracy untouched.
deepregression: a Flexible Neural Network Framework for Semi-Structured Deep Distributional Regression
Apr 06, 2021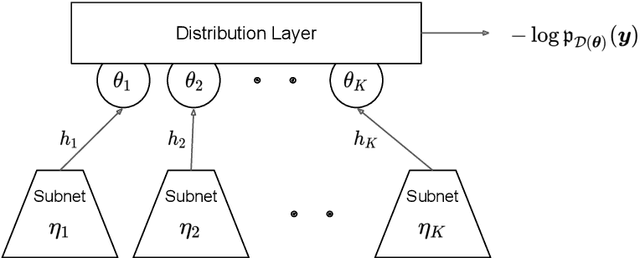
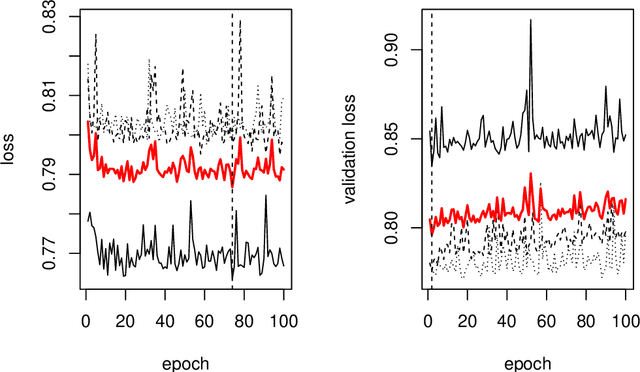
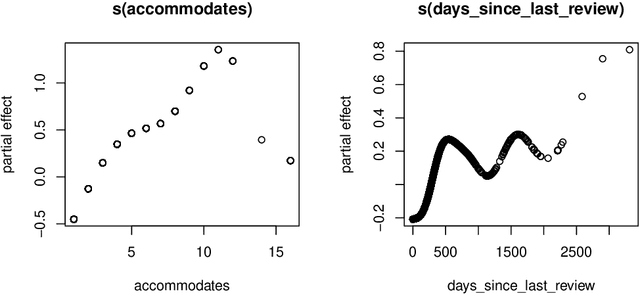
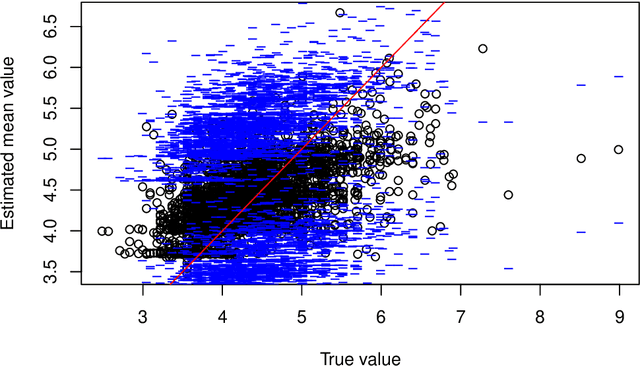
Abstract:This paper describes the implementation of semi-structured deep distributional regression, a flexible framework to learn distributions based on a combination of additive regression models and deep neural networks. deepregression is implemented in both R and Python, using the deep learning libraries TensorFlow and PyTorch, respectively. The implementation consists of (1) a modular neural network building system for the combination of various statistical and deep learning approaches, (2) an orthogonalization cell to allow for an interpretable combination of different subnetworks as well as (3) pre-processing steps necessary to initialize such models. The software package allows to define models in a user-friendly manner using distribution definitions via a formula environment that is inspired by classical statistical model frameworks such as mgcv. The packages' modular design and functionality provides a unique resource for rapid and reproducible prototyping of complex statistical and deep learning models while simultaneously retaining the indispensable interpretability of classical statistical models.
Patient-specific virtual spine straightening and vertebra inpainting: An automatic framework for osteoplasty planning
Mar 23, 2021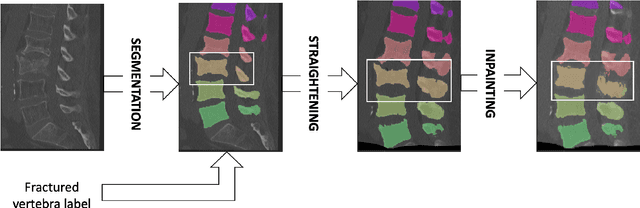

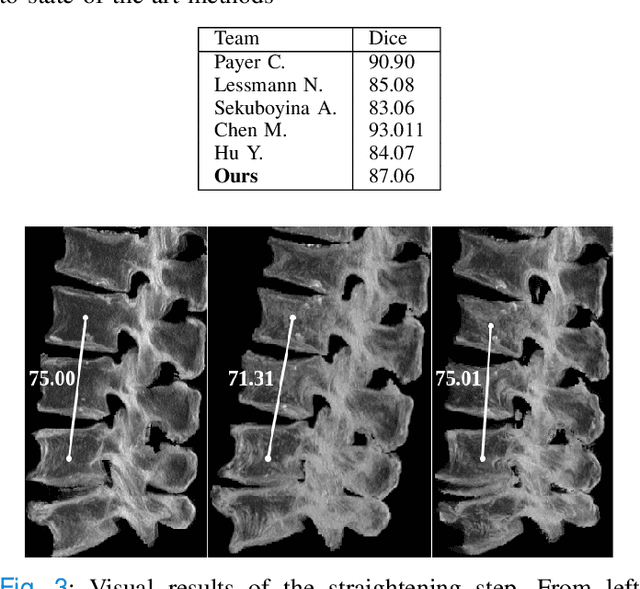
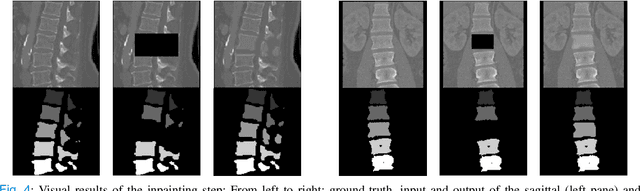
Abstract:Symptomatic spinal vertebral compression fractures (VCFs) often require osteoplasty treatment. A cement-like material is injected into the bone to stabilize the fracture, restore the vertebral body height and alleviate pain. Leakage is a common complication and may occur due to too much cement being injected. In this work, we propose an automated patient-specific framework that can allow physicians to calculate an upper bound of cement for the injection and estimate the optimal outcome of osteoplasty. The framework uses the patient CT scan and the fractured vertebra label to build a virtual healthy spine using a high-level approach. Firstly, the fractured spine is segmented with a three-step Convolution Neural Network (CNN) architecture. Next, a per-vertebra rigid registration to a healthy spine atlas restores its curvature. Finally, a GAN-based inpainting approach replaces the fractured vertebra with an estimation of its original shape. Based on this outcome, we then estimate the maximum amount of bone cement for injection. We evaluate our framework by comparing the virtual vertebrae volumes of ten patients to their healthy equivalent and report an average error of 3.88$\pm$7.63\%. The presented pipeline offers a first approach to a personalized automatic high-level framework for planning osteoplasty procedures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge