Alejandro Granados
From Pre- to Intra-operative MRI: Predicting Brain Shift in Temporal Lobe Resection for Epilepsy Surgery
Feb 03, 2026Abstract:Introduction: In neurosurgery, image-guided Neurosurgery Systems (IGNS) highly rely on preoperative brain magnetic resonance images (MRI) to assist surgeons in locating surgical targets and determining surgical paths. However, brain shift invalidates the preoperative MRI after dural opening. Updated intraoperative brain MRI with brain shift compensation is crucial for enhancing the precision of neuronavigation systems and ensuring the optimal outcome of surgical interventions. Methodology: We propose NeuralShift, a U-Net-based model that predicts brain shift entirely from pre-operative MRI for patients undergoing temporal lobe resection. We evaluated our results using Target Registration Errors (TREs) computed on anatomical landmarks located on the resection side and along the midline, and DICE scores comparing predicted intraoperative masks with masks derived from intraoperative MRI. Results: Our experimental results show that our model can predict the global deformation of the brain (DICE of 0.97) with accurate local displacements (achieve landmark TRE as low as 1.12 mm), compensating for large brain shifts during temporal lobe removal neurosurgery. Conclusion: Our proposed model is capable of predicting the global deformation of the brain during temporal lobe resection using only preoperative images, providing potential opportunities to the surgical team to increase safety and efficiency of neurosurgery and better outcomes to patients. Our contributions will be publicly available after acceptance in https://github.com/SurgicalDataScienceKCL/NeuralShift.
Reinforcement Learning for Safe Autonomous Two Device Navigation of Cerebral Vessels in Mechanical Thrombectomy
Mar 31, 2025Abstract:Purpose: Autonomous systems in mechanical thrombectomy (MT) hold promise for reducing procedure times, minimizing radiation exposure, and enhancing patient safety. However, current reinforcement learning (RL) methods only reach the carotid arteries, are not generalizable to other patient vasculatures, and do not consider safety. We propose a safe dual-device RL algorithm that can navigate beyond the carotid arteries to cerebral vessels. Methods: We used the Simulation Open Framework Architecture to represent the intricacies of cerebral vessels, and a modified Soft Actor-Critic RL algorithm to learn, for the first time, the navigation of micro-catheters and micro-guidewires. We incorporate patient safety metrics into our reward function by integrating guidewire tip forces. Inverse RL is used with demonstrator data on 12 patient-specific vascular cases. Results: Our simulation demonstrates successful autonomous navigation within unseen cerebral vessels, achieving a 96% success rate, 7.0s procedure time, and 0.24 N mean forces, well below the proposed 1.5 N vessel rupture threshold. Conclusion: To the best of our knowledge, our proposed autonomous system for MT two-device navigation reaches cerebral vessels, considers safety, and is generalizable to unseen patient-specific cases for the first time. We envisage future work will extend the validation to vasculatures of different complexity and on in vitro models. While our contributions pave the way towards deploying agents in clinical settings, safety and trustworthiness will be crucial elements to consider when proposing new methodology.
UltraFlwr -- An Efficient Federated Medical and Surgical Object Detection Framework
Mar 19, 2025Abstract:Object detection shows promise for medical and surgical applications such as cell counting and tool tracking. However, its faces multiple real-world edge deployment challenges including limited high-quality annotated data, data sharing restrictions, and computational constraints. In this work, we introduce UltraFlwr, a framework for federated medical and surgical object detection. By leveraging Federated Learning (FL), UltraFlwr enables decentralized model training across multiple sites without sharing raw data. To further enhance UltraFlwr's efficiency, we propose YOLO-PA, a set of novel Partial Aggregation (PA) strategies specifically designed for YOLO models in FL. YOLO-PA significantly reduces communication overhead by up to 83% per round while maintaining performance comparable to Full Aggregation (FA) strategies. Our extensive experiments on BCCD and m2cai16-tool-locations datasets demonstrate that YOLO-PA not only provides better client models compared to client-wise centralized training and FA strategies, but also facilitates efficient training and deployment across resource-constrained edge devices. Further, we also establish one of the first benchmarks in federated medical and surgical object detection. This paper advances the feasibility of training and deploying detection models on the edge, making federated object detection more practical for time-critical and resource-constrained medical and surgical applications. UltraFlwr is publicly available at https://github.com/KCL-BMEIS/UltraFlwr.
SWAG: Long-term Surgical Workflow Prediction with Generative-based Anticipation
Dec 25, 2024
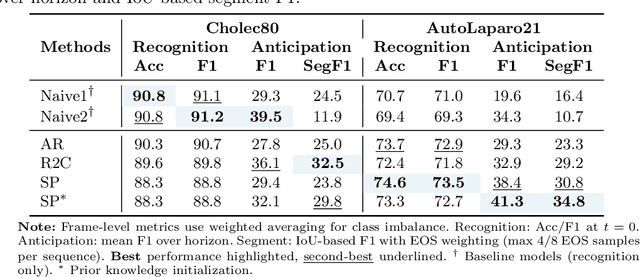
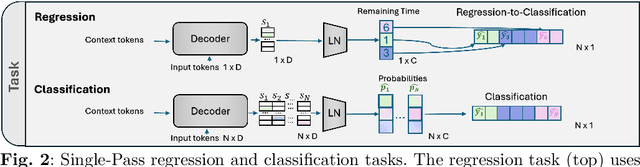
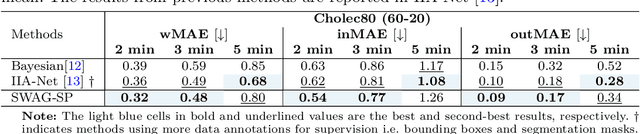
Abstract:While existing recognition approaches excel at identifying current surgical phases, they provide limited foresight into future procedural steps, restricting their intraoperative utility. Similarly, current anticipation methods are constrained to predicting short-term events or singular future occurrences, neglecting the dynamic and sequential nature of surgical workflows. To address these limitations, we propose SWAG (Surgical Workflow Anticipative Generation), a unified framework for phase recognition and long-term anticipation of surgical workflows. SWAG employs two generative decoding methods -- single-pass (SP) and auto-regressive (AR) -- to predict sequences of future surgical phases. A novel prior knowledge embedding mechanism enhances the accuracy of anticipatory predictions. The framework addresses future phase classification and remaining time regression tasks. Additionally, a regression-to-classification (R2C) method is introduced to map continuous predictions to discrete temporal segments. SWAG's performance was evaluated on the Cholec80 and AutoLaparo21 datasets. The single-pass classification model with prior knowledge embeddings (SWAG-SP\*) achieved 53.5\% accuracy in 15-minute anticipation on AutoLaparo21, while the R2C model reached 60.8\% accuracy on Cholec80. SWAG's single-pass regression approach outperformed existing methods for remaining time prediction, achieving weighted mean absolute errors of 0.32 and 0.48 minutes for 2- and 3-minute horizons, respectively. SWAG demonstrates versatility across classification and regression tasks, offering robust tools for real-time surgical workflow anticipation. By unifying recognition and anticipatory capabilities, SWAG provides actionable predictions to enhance intraoperative decision-making.
Autonomous navigation of catheters and guidewires in mechanical thrombectomy using inverse reinforcement learning
Jun 18, 2024Abstract:Purpose: Autonomous navigation of catheters and guidewires can enhance endovascular surgery safety and efficacy, reducing procedure times and operator radiation exposure. Integrating tele-operated robotics could widen access to time-sensitive emergency procedures like mechanical thrombectomy (MT). Reinforcement learning (RL) shows potential in endovascular navigation, yet its application encounters challenges without a reward signal. This study explores the viability of autonomous navigation in MT vasculature using inverse RL (IRL) to leverage expert demonstrations. Methods: This study established a simulation-based training and evaluation environment for MT navigation. We used IRL to infer reward functions from expert behaviour when navigating a guidewire and catheter. We utilized soft actor-critic to train models with various reward functions and compared their performance in silico. Results: We demonstrated feasibility of navigation using IRL. When evaluating single versus dual device (i.e. guidewire versus catheter and guidewire) tracking, both methods achieved high success rates of 95% and 96%, respectively. Dual-tracking, however, utilized both devices mimicking an expert. A success rate of 100% and procedure time of 22.6 s were obtained when training with a reward function obtained through reward shaping. This outperformed a dense reward function (96%, 24.9 s) and an IRL-derived reward function (48%, 59.2 s). Conclusions: We have contributed to the advancement of autonomous endovascular intervention navigation, particularly MT, by employing IRL. The results underscore the potential of using reward shaping to train models, offering a promising avenue for enhancing the accessibility and precision of MT. We envisage that future research can extend our methodology to diverse anatomical structures to enhance generalizability.
* Abstract shortened for arXiv character limit
Artificial Intelligence in the Autonomous Navigation of Endovascular Interventions: A Systematic Review
May 06, 2024



Abstract:Purpose: Autonomous navigation of devices in endovascular interventions can decrease operation times, improve decision-making during surgery, and reduce operator radiation exposure while increasing access to treatment. This systematic review explores recent literature to assess the impact, challenges, and opportunities artificial intelligence (AI) has for the autonomous endovascular intervention navigation. Methods: PubMed and IEEEXplore databases were queried. Eligibility criteria included studies investigating the use of AI in enabling the autonomous navigation of catheters/guidewires in endovascular interventions. Following PRISMA, articles were assessed using QUADAS-2. PROSPERO: CRD42023392259. Results: Among 462 studies, fourteen met inclusion criteria. Reinforcement learning (9/14, 64%) and learning from demonstration (7/14, 50%) were used as data-driven models for autonomous navigation. Studies predominantly utilised physical phantoms (10/14, 71%) and in silico (4/14, 29%) models. Experiments within or around the blood vessels of the heart were reported by the majority of studies (10/14, 71%), while simple non-anatomical vessel platforms were used in three studies (3/14, 21%), and the porcine liver venous system in one study. We observed that risk of bias and poor generalisability were present across studies. No procedures were performed on patients in any of the studies reviewed. Studies lacked patient selection criteria, reference standards, and reproducibility, resulting in low clinical evidence levels. Conclusions: AI's potential in autonomous endovascular navigation is promising, but in an experimental proof-of-concept stage, with a technology readiness level of 3. We highlight that reference standards with well-identified performance metrics are crucial to allow for comparisons of data-driven algorithms proposed in the years to come.
* Abstract shortened for arXiv character limit
DDSB: An Unsupervised and Training-free Method for Phase Detection in Echocardiography
Mar 19, 2024



Abstract:Accurate identification of End-Diastolic (ED) and End-Systolic (ES) frames is key for cardiac function assessment through echocardiography. However, traditional methods face several limitations: they require extensive amounts of data, extensive annotations by medical experts, significant training resources, and often lack robustness. Addressing these challenges, we proposed an unsupervised and training-free method, our novel approach leverages unsupervised segmentation to enhance fault tolerance against segmentation inaccuracies. By identifying anchor points and analyzing directional deformation, we effectively reduce dependence on the accuracy of initial segmentation images and enhance fault tolerance, all while improving robustness. Tested on Echo-dynamic and CAMUS datasets, our method achieves comparable accuracy to learning-based models without their associated drawbacks. The code is available at https://github.com/MRUIL/DDSB
Rethinking Low-quality Optical Flow in Unsupervised Surgical Instrument Segmentation
Mar 15, 2024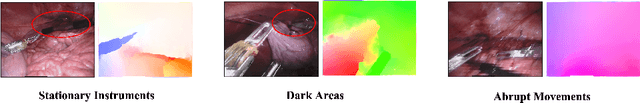
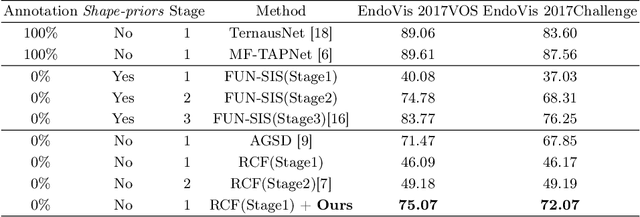
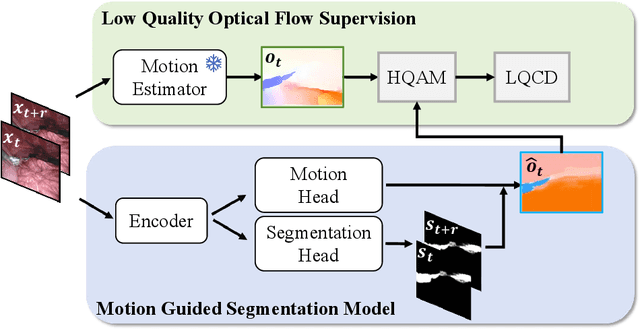
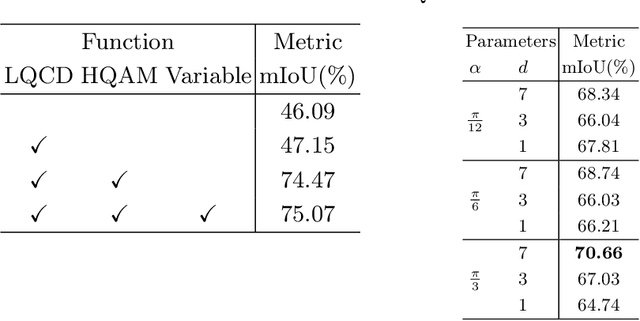
Abstract:Video-based surgical instrument segmentation plays an important role in robot-assisted surgeries. Unlike supervised settings, unsupervised segmentation relies heavily on motion cues, which are challenging to discern due to the typically lower quality of optical flow in surgical footage compared to natural scenes. This presents a considerable burden for the advancement of unsupervised segmentation techniques. In our work, we address the challenge of enhancing model performance despite the inherent limitations of low-quality optical flow. Our methodology employs a three-pronged approach: extracting boundaries directly from the optical flow, selectively discarding frames with inferior flow quality, and employing a fine-tuning process with variable frame rates. We thoroughly evaluate our strategy on the EndoVis2017 VOS dataset and Endovis2017 Challenge dataset, where our model demonstrates promising results, achieving a mean Intersection-over-Union (mIoU) of 0.75 and 0.72, respectively. Our findings suggest that our approach can greatly decrease the need for manual annotations in clinical environments and may facilitate the annotation process for new datasets. The code is available at https://github.com/wpr1018001/Rethinking-Low-quality-Optical-Flow.git
SuPRA: Surgical Phase Recognition and Anticipation for Intra-Operative Planning
Mar 10, 2024



Abstract:Intra-operative recognition of surgical phases holds significant potential for enhancing real-time contextual awareness in the operating room. However, we argue that online recognition, while beneficial, primarily lends itself to post-operative video analysis due to its limited direct impact on the actual surgical decisions and actions during ongoing procedures. In contrast, we contend that the prediction and anticipation of surgical phases are inherently more valuable for intra-operative assistance, as they can meaningfully influence a surgeon's immediate and long-term planning by providing foresight into future steps. To address this gap, we propose a dual approach that simultaneously recognises the current surgical phase and predicts upcoming ones, thus offering comprehensive intra-operative assistance and guidance on the expected remaining workflow. Our novel method, Surgical Phase Recognition and Anticipation (SuPRA), leverages past and current information for accurate intra-operative phase recognition while using future segments for phase prediction. This unified approach challenges conventional frameworks that treat these objectives separately. We have validated SuPRA on two reputed datasets, Cholec80 and AutoLaparo21, where it demonstrated state-of-the-art performance with recognition accuracies of 91.8% and 79.3%, respectively. Additionally, we introduce and evaluate our model using new segment-level evaluation metrics, namely Edit and F1 Overlap scores, for a more temporal assessment of segment classification. In conclusion, SuPRA presents a new multi-task approach that paves the way for improved intra-operative assistance through surgical phase recognition and prediction of future events.
ArcSin: Adaptive ranged cosine Similarity injected noise for Language-Driven Visual Tasks
Feb 27, 2024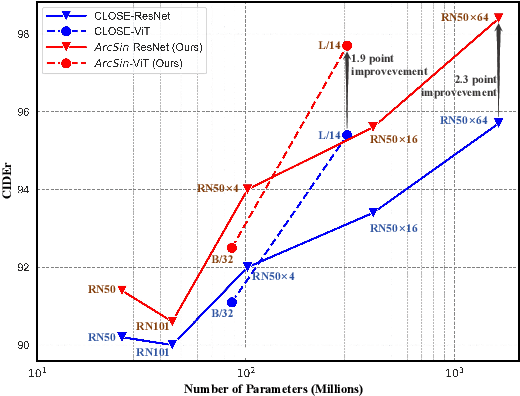
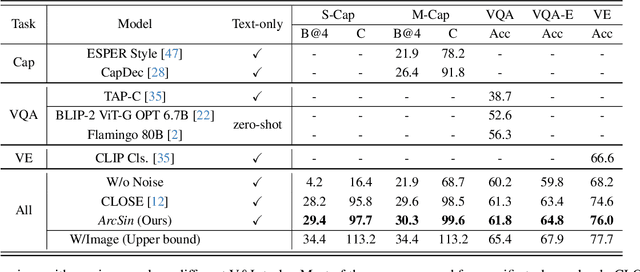
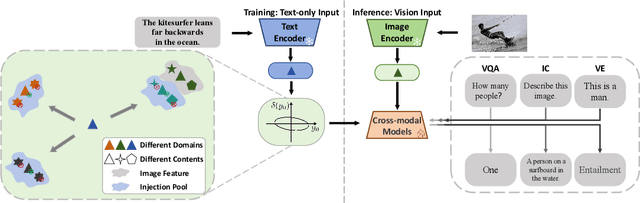

Abstract:In this study, we address the challenging task of bridging the modality gap between learning from language and inference for visual tasks, including Visual Question Answering (VQA), Image Captioning (IC) and Visual Entailment (VE). We train models for these tasks in a zero-shot cross-modal transfer setting, a domain where the previous state-of-the-art method relied on the fixed scale noise injection, often compromising the semantic content of the original modality embedding. To combat it, we propose a novel method called Adaptive ranged cosine Similarity injected noise (ArcSin). First, we introduce an innovative adaptive noise scale that effectively generates the textual elements with more variability while preserving the original text feature's integrity. Second, a similarity pool strategy is employed, expanding the domain generalization potential by broadening the overall noise scale. This dual strategy effectively widens the scope of the original domain while safeguarding content integrity. Our empirical results demonstrate that these models closely rival those trained on images in terms of performance. Specifically, our method exhibits substantial improvements over the previous state-of-the-art, achieving gains of 1.9 and 1.1 CIDEr points in S-Cap and M-Cap, respectively. Additionally, we observe increases of 1.5 percentage points (pp), 1.4 pp, and 1.4 pp in accuracy for VQA, VQA-E, and VE, respectively, pushing the boundaries of what is achievable within the constraints of image-trained model benchmarks. The code will be released.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge