Wolfgang Reiter
SurgT challenge: Benchmark of Soft-Tissue Trackers for Robotic Surgery
Feb 28, 2023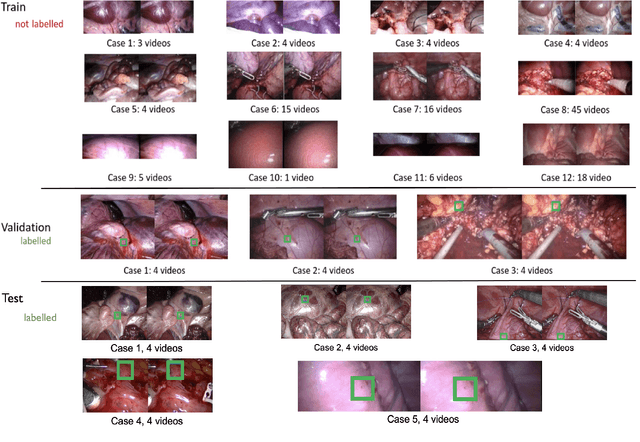
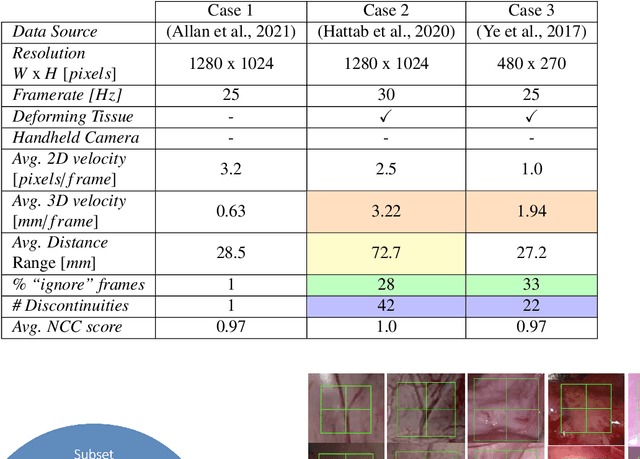
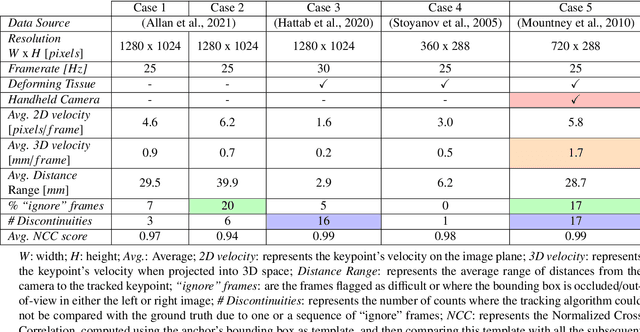
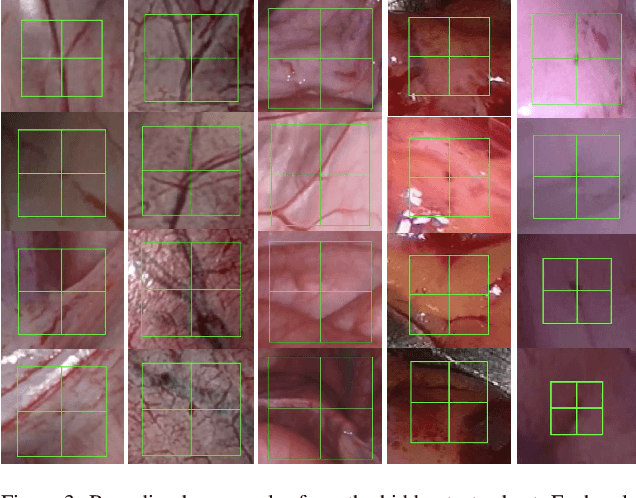
Abstract:This paper introduces the "SurgT: Surgical Tracking" challenge which was organised in conjunction with the 25th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2022). There were two purposes for the creation of this challenge: (1) the establishment of the first standardised benchmark for the research community to assess soft-tissue trackers; and (2) to encourage the development of unsupervised deep learning methods, given the lack of annotated data in surgery. A dataset of 157 stereo endoscopic videos from 20 clinical cases, along with stereo camera calibration parameters, have been provided. The participants were tasked with the development of algorithms to track a bounding box on stereo endoscopic videos. At the end of the challenge, the developed methods were assessed on a previously hidden test subset. This assessment uses benchmarking metrics that were purposely developed for this challenge and are now available online. The teams were ranked according to their Expected Average Overlap (EAO) score, which is a weighted average of the Intersection over Union (IoU) scores. The performance evaluation study verifies the efficacy of unsupervised deep learning algorithms in tracking soft-tissue. The best-performing method achieved an EAO score of 0.583 in the test subset. The dataset and benchmarking tool created for this challenge have been made publicly available. This challenge is expected to contribute to the development of autonomous robotic surgery and other digital surgical technologies.
CholecTriplet2022: Show me a tool and tell me the triplet -- an endoscopic vision challenge for surgical action triplet detection
Feb 13, 2023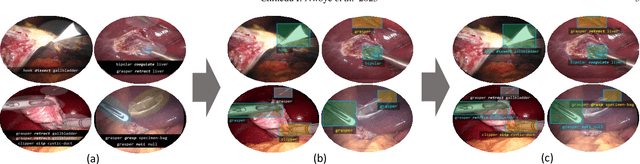
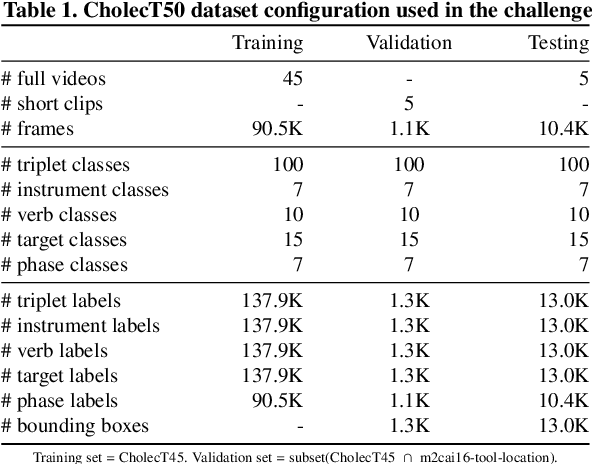
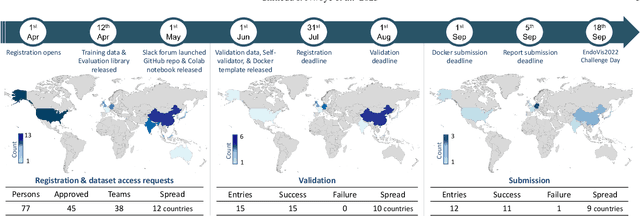
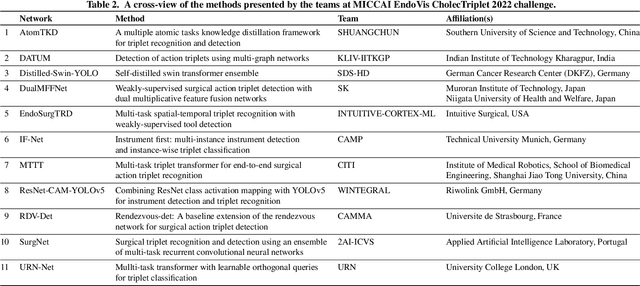
Abstract:Formalizing surgical activities as triplets of the used instruments, actions performed, and target anatomies is becoming a gold standard approach for surgical activity modeling. The benefit is that this formalization helps to obtain a more detailed understanding of tool-tissue interaction which can be used to develop better Artificial Intelligence assistance for image-guided surgery. Earlier efforts and the CholecTriplet challenge introduced in 2021 have put together techniques aimed at recognizing these triplets from surgical footage. Estimating also the spatial locations of the triplets would offer a more precise intraoperative context-aware decision support for computer-assisted intervention. This paper presents the CholecTriplet2022 challenge, which extends surgical action triplet modeling from recognition to detection. It includes weakly-supervised bounding box localization of every visible surgical instrument (or tool), as the key actors, and the modeling of each tool-activity in the form of <instrument, verb, target> triplet. The paper describes a baseline method and 10 new deep learning algorithms presented at the challenge to solve the task. It also provides thorough methodological comparisons of the methods, an in-depth analysis of the obtained results, their significance, and useful insights for future research directions and applications in surgery.
Comparative Validation of Machine Learning Algorithms for Surgical Workflow and Skill Analysis with the HeiChole Benchmark
Sep 30, 2021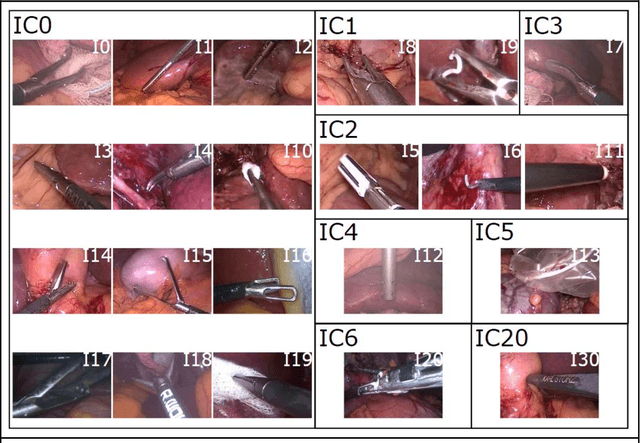
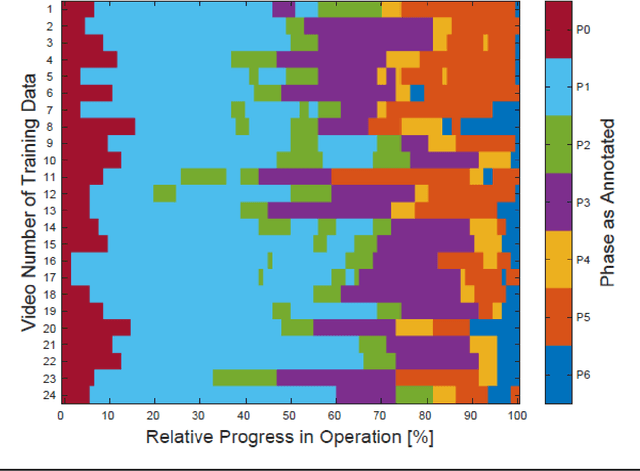
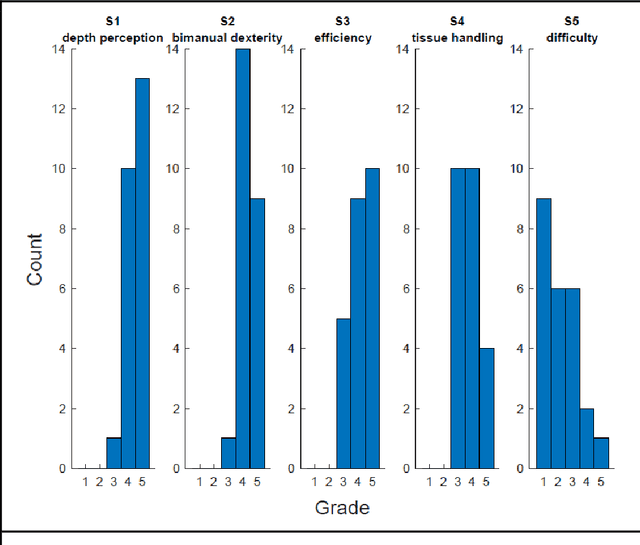
Abstract:PURPOSE: Surgical workflow and skill analysis are key technologies for the next generation of cognitive surgical assistance systems. These systems could increase the safety of the operation through context-sensitive warnings and semi-autonomous robotic assistance or improve training of surgeons via data-driven feedback. In surgical workflow analysis up to 91% average precision has been reported for phase recognition on an open data single-center dataset. In this work we investigated the generalizability of phase recognition algorithms in a multi-center setting including more difficult recognition tasks such as surgical action and surgical skill. METHODS: To achieve this goal, a dataset with 33 laparoscopic cholecystectomy videos from three surgical centers with a total operation time of 22 hours was created. Labels included annotation of seven surgical phases with 250 phase transitions, 5514 occurences of four surgical actions, 6980 occurences of 21 surgical instruments from seven instrument categories and 495 skill classifications in five skill dimensions. The dataset was used in the 2019 Endoscopic Vision challenge, sub-challenge for surgical workflow and skill analysis. Here, 12 teams submitted their machine learning algorithms for recognition of phase, action, instrument and/or skill assessment. RESULTS: F1-scores were achieved for phase recognition between 23.9% and 67.7% (n=9 teams), for instrument presence detection between 38.5% and 63.8% (n=8 teams), but for action recognition only between 21.8% and 23.3% (n=5 teams). The average absolute error for skill assessment was 0.78 (n=1 team). CONCLUSION: Surgical workflow and skill analysis are promising technologies to support the surgical team, but are not solved yet, as shown by our comparison of algorithms. This novel benchmark can be used for comparable evaluation and validation of future work.
MIcro-Surgical Anastomose Workflow recognition challenge report
Mar 24, 2021



Abstract:The "MIcro-Surgical Anastomose Workflow recognition on training sessions" (MISAW) challenge provided a data set of 27 sequences of micro-surgical anastomosis on artificial blood vessels. This data set was composed of videos, kinematics, and workflow annotations described at three different granularity levels: phase, step, and activity. The participants were given the option to use kinematic data and videos to develop workflow recognition models. Four tasks were proposed to the participants: three of them were related to the recognition of surgical workflow at three different granularity levels, while the last one addressed the recognition of all granularity levels in the same model. One ranking was made for each task. We used the average application-dependent balanced accuracy (AD-Accuracy) as the evaluation metric. This takes unbalanced classes into account and it is more clinically relevant than a frame-by-frame score. Six teams, including a non-competing team, participated in at least one task. All models employed deep learning models, such as CNN or RNN. The best models achieved more than 95% AD-Accuracy for phase recognition, 80% for step recognition, 60% for activity recognition, and 75% for all granularity levels. For high levels of granularity (i.e., phases and steps), the best models had a recognition rate that may be sufficient for applications such as prediction of remaining surgical time or resource management. However, for activities, the recognition rate was still low for applications that can be employed clinically. The MISAW data set is publicly available to encourage further research in surgical workflow recognition. It can be found at www.synapse.org/MISAW
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge