Wenjia Xie
Machine Learning Techniques for Multifactor Analysis of National Carbon Dioxide Emissions
Mar 19, 2025Abstract:This paper presents a comprehensive study leveraging Support Vector Machine (SVM) regression and Principal Component Regression (PCR) to analyze carbon dioxide emissions in a global dataset of 62 countries and their dependence on idiosyncratic, country-specific parameters. The objective is to understand the factors contributing to carbon dioxide emissions and identify the most predictive elements. The analysis provides country-specific emission estimates, highlighting diverse national trajectories and pinpointing areas for targeted interventions in climate change mitigation, sustainable development, and the growing carbon credit markets and green finance sector. The study aims to support policymaking with accurate representations of carbon dioxide emissions, offering nuanced information for formulating effective strategies to address climate change while informing initiatives related to carbon trading and environmentally sustainable investments.
Scaling New Frontiers: Insights into Large Recommendation Models
Dec 01, 2024



Abstract:Recommendation systems are essential for filtering data and retrieving relevant information across various applications. Recent advancements have seen these systems incorporate increasingly large embedding tables, scaling up to tens of terabytes for industrial use. However, the expansion of network parameters in traditional recommendation models has plateaued at tens of millions, limiting further benefits from increased embedding parameters. Inspired by the success of large language models (LLMs), a new approach has emerged that scales network parameters using innovative structures, enabling continued performance improvements. A significant development in this area is Meta's generative recommendation model HSTU, which illustrates the scaling laws of recommendation systems by expanding parameters to thousands of billions. This new paradigm has achieved substantial performance gains in online experiments. In this paper, we aim to enhance the understanding of scaling laws by conducting comprehensive evaluations of large recommendation models. Firstly, we investigate the scaling laws across different backbone architectures of the large recommendation models. Secondly, we conduct comprehensive ablation studies to explore the origins of these scaling laws. We then further assess the performance of HSTU, as the representative of large recommendation models, on complex user behavior modeling tasks to evaluate its applicability. Notably, we also analyze its effectiveness in ranking tasks for the first time. Finally, we offer insights into future directions for large recommendation models. Supplementary materials for our research are available on GitHub at https://github.com/USTC-StarTeam/Large-Recommendation-Models.
Breaking Determinism: Fuzzy Modeling of Sequential Recommendation Using Discrete State Space Diffusion Model
Oct 31, 2024



Abstract:Sequential recommendation (SR) aims to predict items that users may be interested in based on their historical behavior sequences. We revisit SR from a novel information-theoretic perspective and find that conventional sequential modeling methods fail to adequately capture the randomness and unpredictability of user behavior. Inspired by fuzzy information processing theory, this paper introduces the DDSR model, which uses fuzzy sets of interaction sequences to overcome the limitations and better capture the evolution of users' real interests. Formally based on diffusion transition processes in discrete state spaces, which is unlike common diffusion models such as DDPM that operate in continuous domains. It is better suited for discrete data, using structured transitions instead of arbitrary noise introduction to avoid information loss. Additionally, to address the inefficiency of matrix transformations due to the vast discrete space, we use semantic labels derived from quantization or RQ-VAE to replace item IDs, enhancing efficiency and improving cold start issues. Testing on three public benchmark datasets shows that DDSR outperforms existing state-of-the-art methods in various settings, demonstrating its potential and effectiveness in handling SR tasks.
Enabling Collaborative Clinical Diagnosis of Infectious Keratitis by Integrating Expert Knowledge and Interpretable Data-driven Intelligence
Jan 14, 2024Abstract:Although data-driven artificial intelligence (AI) in medical image diagnosis has shown impressive performance in silico, the lack of interpretability makes it difficult to incorporate the "black box" into clinicians' workflows. To make the diagnostic patterns learned from data understandable by clinicians, we develop an interpretable model, knowledge-guided diagnosis model (KGDM), that provides a visualized reasoning process containing AI-based biomarkers and retrieved cases that with the same diagnostic patterns. It embraces clinicians' prompts into the interpreted reasoning through human-AI interaction, leading to potentially enhanced safety and more accurate predictions. This study investigates the performance, interpretability, and clinical utility of KGDM in the diagnosis of infectious keratitis (IK), which is the leading cause of corneal blindness. The classification performance of KGDM is evaluated on a prospective validation dataset, an external testing dataset, and an publicly available testing dataset. The diagnostic odds ratios (DOR) of the interpreted AI-based biomarkers are effective, ranging from 3.011 to 35.233 and exhibit consistent diagnostic patterns with clinic experience. Moreover, a human-AI collaborative diagnosis test is conducted and the participants with collaboration achieved a performance exceeding that of both humans and AI. By synergistically integrating interpretability and interaction, this study facilitates the convergence of clinicians' expertise and data-driven intelligence. The promotion of inexperienced ophthalmologists with the aid of AI-based biomarkers, as well as increased AI prediction by intervention from experienced ones, demonstrate a promising diagnostic paradigm for infectious keratitis using KGDM, which holds the potential for extension to other diseases where experienced medical practitioners are limited and the safety of AI is concerned.
Deep Sequential Feature Learning in Clinical Image Classification of Infectious Keratitis
Jun 04, 2020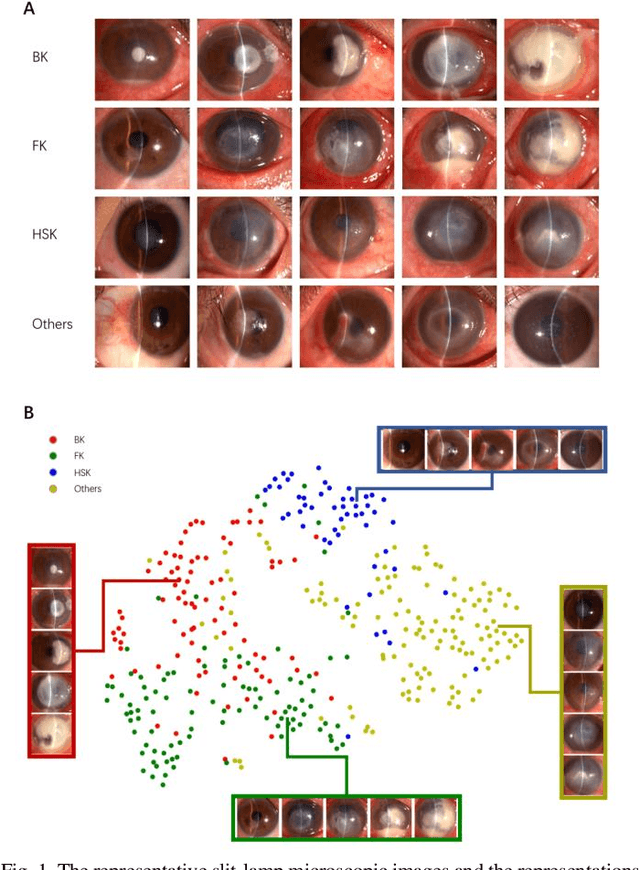
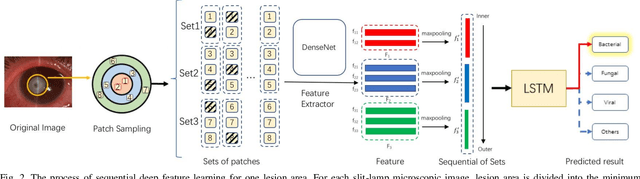
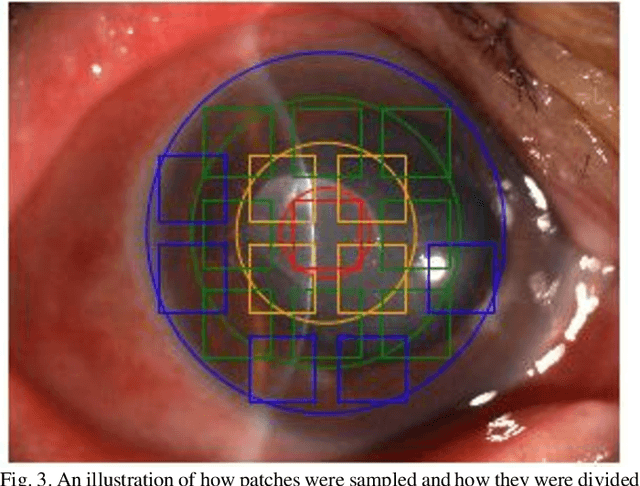
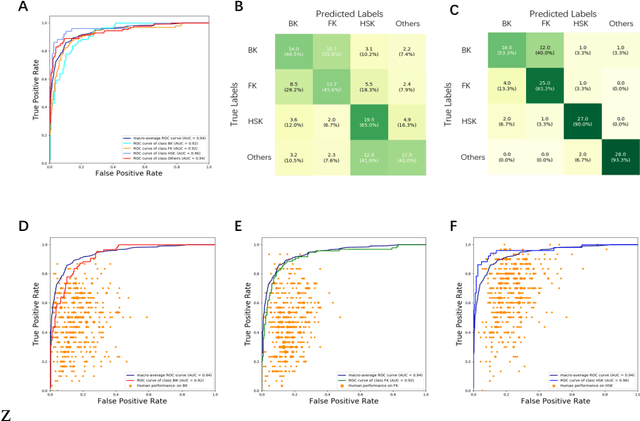
Abstract:Infectious keratitis is the most common entities of corneal diseases, in which pathogen grows in the cornea leading to inflammation and destruction of the corneal tissues. Infectious keratitis is a medical emergency, for which a rapid and accurate diagnosis is needed for speedy initiation of prompt and precise treatment to halt the disease progress and to limit the extent of corneal damage; otherwise it may develop sight-threatening and even eye-globe-threatening condition. In this paper, we propose a sequential-level deep learning model to effectively discriminate the distinction and subtlety of infectious corneal disease via the classification of clinical images. In this approach, we devise an appropriate mechanism to preserve the spatial structures of clinical images and disentangle the informative features for clinical image classification of infectious keratitis. In competition with 421 ophthalmologists, the performance of the proposed sequential-level deep model achieved 80.00% diagnostic accuracy, far better than the 49.27% diagnostic accuracy achieved by ophthalmologists over 120 test images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge