Victor Greiff
Best practices for machine learning in antibody discovery and development
Dec 16, 2023



Abstract:Over the past 40 years, the discovery and development of therapeutic antibodies to treat disease has become common practice. However, as therapeutic antibody constructs are becoming more sophisticated (e.g., multi-specifics), conventional approaches to optimisation are increasingly inefficient. Machine learning (ML) promises to open up an in silico route to antibody discovery and help accelerate the development of drug products using a reduced number of experiments and hence cost. Over the past few years, we have observed rapid developments in the field of ML-guided antibody discovery and development (D&D). However, many of the results are difficult to compare or hard to assess for utility by other experts in the field due to the high diversity in the datasets and evaluation techniques and metrics that are across industry and academia. This limitation of the literature curtails the broad adoption of ML across the industry and slows down overall progress in the field, highlighting the need to develop standards and guidelines that may help improve the reproducibility of ML models across different research groups. To address these challenges, we set out in this perspective to critically review current practices, explain common pitfalls, and clearly define a set of method development and evaluation guidelines that can be applied to different types of ML-based techniques for therapeutic antibody D&D. Specifically, we address in an end-to-end analysis, challenges associated with all aspects of the ML process and recommend a set of best practices for each stage.
ImmunoLingo: Linguistics-based formalization of the antibody language
Sep 26, 2022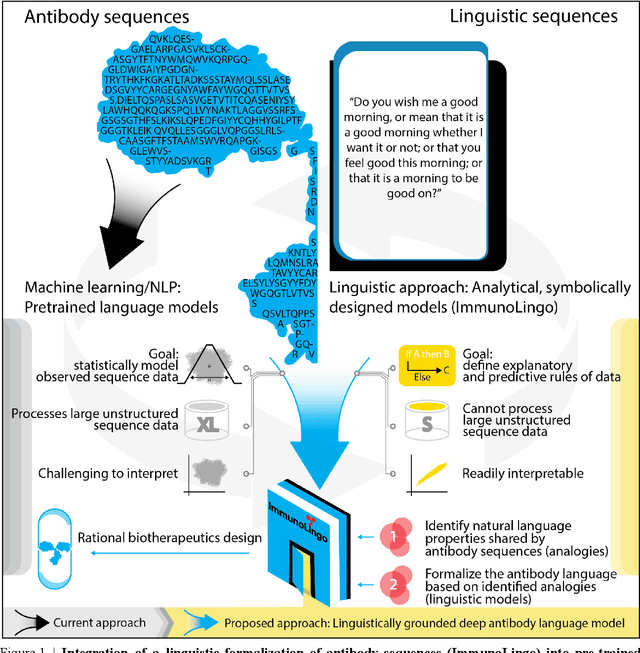
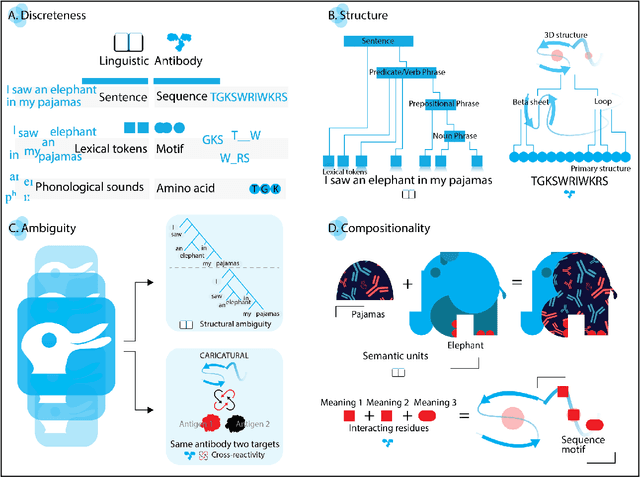
Abstract:Apparent parallels between natural language and biological sequence have led to a recent surge in the application of deep language models (LMs) to the analysis of antibody and other biological sequences. However, a lack of a rigorous linguistic formalization of biological sequence languages, which would define basic components, such as lexicon (i.e., the discrete units of the language) and grammar (i.e., the rules that link sequence well-formedness, structure, and meaning) has led to largely domain-unspecific applications of LMs, which do not take into account the underlying structure of the biological sequences studied. A linguistic formalization, on the other hand, establishes linguistically-informed and thus domain-adapted components for LM applications. It would facilitate a better understanding of how differences and similarities between natural language and biological sequences influence the quality of LMs, which is crucial for the design of interpretable models with extractable sequence-functions relationship rules, such as the ones underlying the antibody specificity prediction problem. Deciphering the rules of antibody specificity is crucial to accelerating rational and in silico biotherapeutic drug design. Here, we formalize the properties of the antibody language and thereby establish not only a foundation for the application of linguistic tools in adaptive immune receptor analysis but also for the systematic immunolinguistic studies of immune receptor specificity in general.
Advancing protein language models with linguistics: a roadmap for improved interpretability
Jul 03, 2022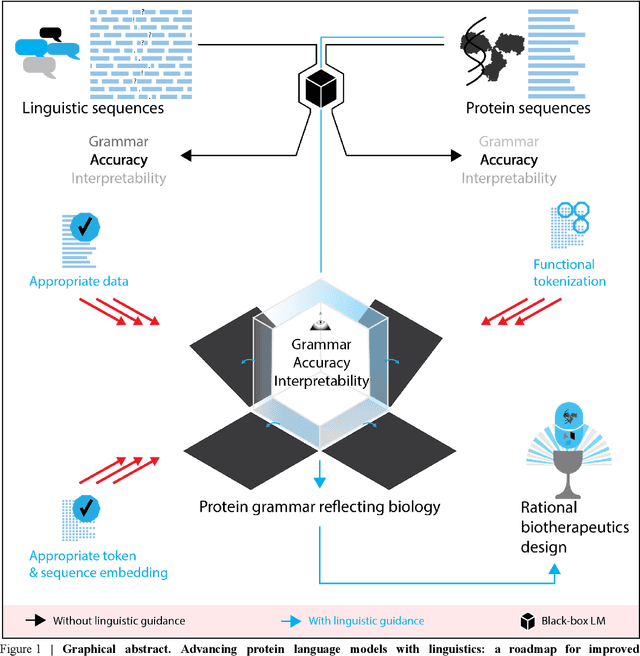
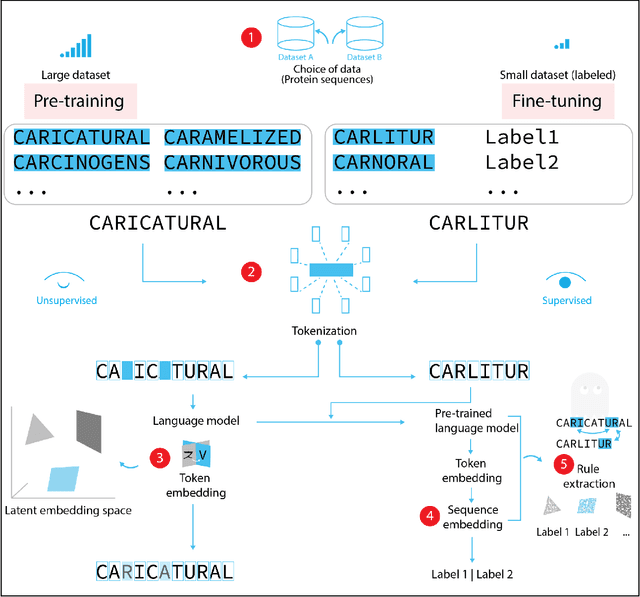
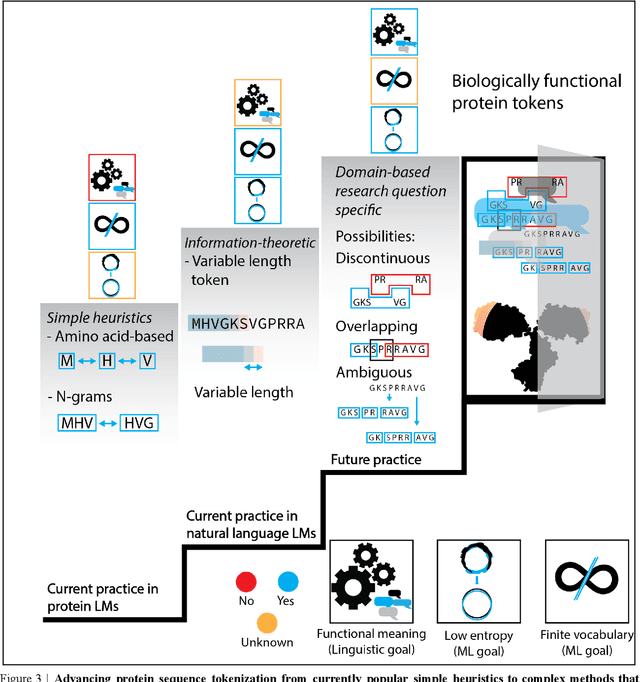
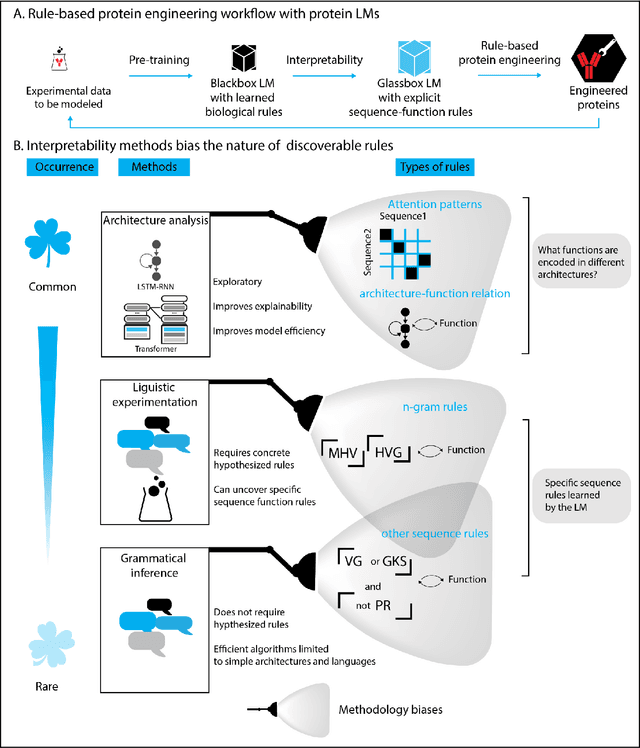
Abstract:Deep neural-network-based language models (LMs) are increasingly applied to large-scale protein sequence data to predict protein function. However, being largely blackbox models and thus challenging to interpret, current protein LM approaches do not contribute to a fundamental understanding of sequence-function mappings, hindering rule-based biotherapeutic drug development. We argue that guidance drawn from linguistics, a field specialized in analytical rule extraction from natural language data, can aid with building more interpretable protein LMs that have learned relevant domain-specific rules. Differences between protein sequence data and linguistic sequence data require the integration of more domain-specific knowledge in protein LMs compared to natural language LMs. Here, we provide a linguistics-based roadmap for protein LM pipeline choices with regard to training data, tokenization, token embedding, sequence embedding, and model interpretation. Combining linguistics with protein LMs enables the development of next-generation interpretable machine learning models with the potential of uncovering the biological mechanisms underlying sequence-function relationships.
Improving generalization of machine learning-identified biomarkers with causal modeling: an investigation into immune receptor diagnostics
Apr 20, 2022



Abstract:Machine learning is increasingly used to discover diagnostic and prognostic biomarkers from high-dimensional molecular data. However, a variety of factors related to experimental design may affect the ability to learn generalizable and clinically applicable diagnostics. Here, we argue that a causal perspective improves the identification of these challenges, and formalizes their relation to the robustness and generalization of machine learning-based diagnostics. To make for a concrete discussion, we focus on a specific, recently established high-dimensional biomarker - adaptive immune receptor repertoires (AIRRs). We discuss how the main biological and experimental factors of the AIRR domain may influence the learned biomarkers and provide easily adjustable simulations of such effects. In conclusion, we find that causal modeling improves machine learning-based biomarker robustness by identifying stable relations between variables and by guiding the adjustment of the relations and variables that vary between populations.
AntBO: Towards Real-World Automated Antibody Design with Combinatorial Bayesian Optimisation
Feb 16, 2022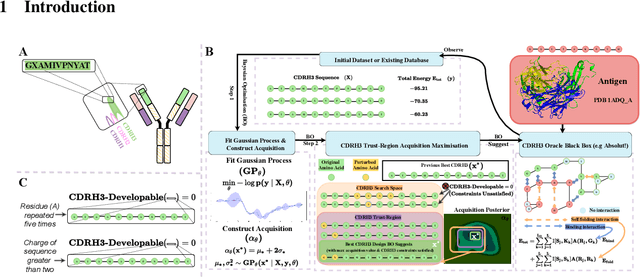
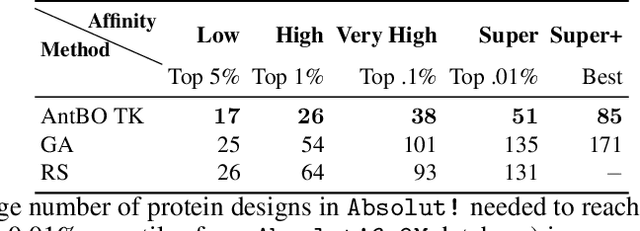
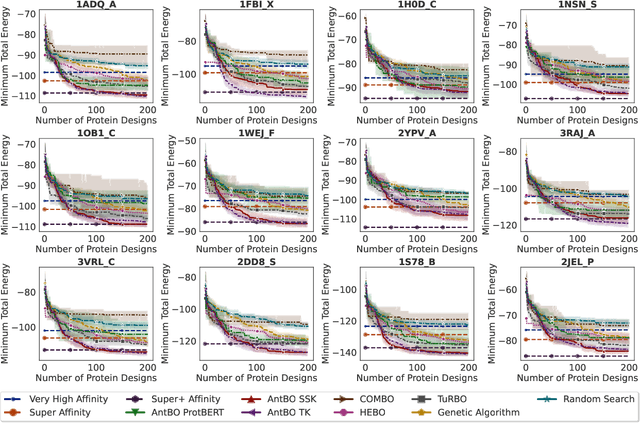
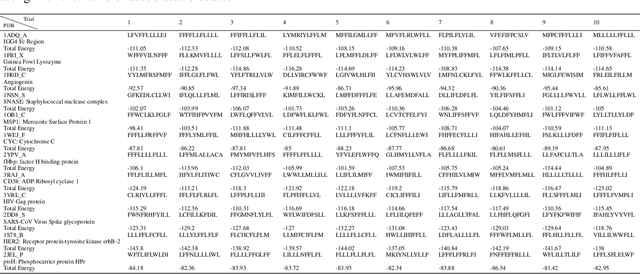
Abstract:Antibodies are canonically Y-shaped multimeric proteins capable of highly specific molecular recognition. The CDRH3 region located at the tip of variable chains of an antibody dominates antigen-binding specificity. Therefore, it is a priority to design optimal antigen-specific CDRH3 regions to develop therapeutic antibodies to combat harmful pathogens. However, the combinatorial nature of CDRH3 sequence space makes it impossible to search for an optimal binding sequence exhaustively and efficiently, especially not experimentally. Here, we present AntBO: a Combinatorial Bayesian Optimisation framework enabling efficient in silico design of the CDRH3 region. Ideally, antibodies should bind to their target antigen and be free from any harmful outcomes. Therefore, we introduce the CDRH3 trust region that restricts the search to sequences with feasible developability scores. To benchmark AntBO, we use the Absolut! software suite as a black-box oracle because it can score the target specificity and affinity of designed antibodies in silico in an unconstrained fashion. The results across 188 antigens demonstrate the benefit of AntBO in designing CDRH3 regions with diverse biophysical properties. In under 200 protein designs, AntBO can suggest antibody sequences that outperform the best binding sequence drawn from 6.9 million experimentally obtained CDRH3s and a commonly used genetic algorithm baseline. Additionally, AntBO finds very-high affinity CDRH3 sequences in only 38 protein designs whilst requiring no domain knowledge. We conclude AntBO brings automated antibody design methods closer to what is practically viable for in vitro experimentation.
Modern Hopfield Networks and Attention for Immune Repertoire Classification
Jul 16, 2020



Abstract:A central mechanism in machine learning is to identify, store, and recognize patterns. How to learn, access, and retrieve such patterns is crucial in Hopfield networks and the more recent transformer architectures. We show that the attention mechanism of transformer architectures is actually the update rule of modern Hopfield networks that can store exponentially many patterns. We exploit this high storage capacity of modern Hopfield networks to solve a challenging multiple instance learning (MIL) problem in computational biology: immune repertoire classification. Accurate and interpretable machine learning methods solving this problem could pave the way towards new vaccines and therapies, which is currently a very relevant research topic intensified by the COVID-19 crisis. Immune repertoire classification based on the vast number of immunosequences of an individual is a MIL problem with an unprecedentedly massive number of instances, two orders of magnitude larger than currently considered problems, and with an extremely low witness rate. In this work, we present our novel method DeepRC that integrates transformer-like attention, or equivalently modern Hopfield networks, into deep learning architectures for massive MIL such as immune repertoire classification. We demonstrate that DeepRC outperforms all other methods with respect to predictive performance on large-scale experiments, including simulated and real-world virus infection data, and enables the extraction of sequence motifs that are connected to a given disease class. Source code and datasets: https://github.com/ml-jku/DeepRC
Hopfield Networks is All You Need
Jul 16, 2020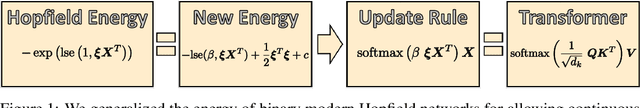
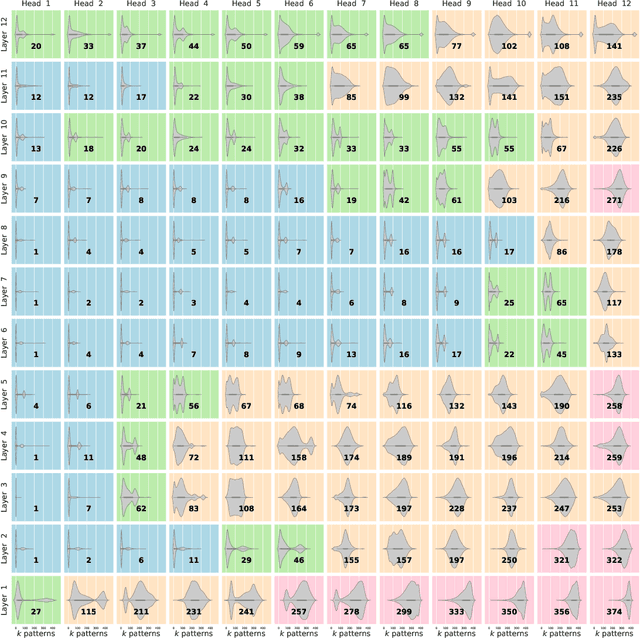
Abstract:We show that the transformer attention mechanism is the update rule of a modern Hopfield network with continuous states. This new Hopfield network can store exponentially (with the dimension) many patterns, converges with one update, and has exponentially small retrieval errors. The number of stored patterns is traded off against convergence speed and retrieval error. The new Hopfield network has three types of energy minima (fixed points of the update): (1) global fixed point averaging over all patterns, (2) metastable states averaging over a subset of patterns, and (3) fixed points which store a single pattern. Transformer and BERT models operate in their first layers preferably in the global averaging regime, while they operate in higher layers in metastable states. The gradient in transformers is maximal for metastable states, is uniformly distributed for global averaging, and vanishes for a fixed point near a stored pattern. Using the Hopfield network interpretation, we analyzed learning of transformer and BERT models. Learning starts with attention heads that average and then most of them switch to metastable states. However, the majority of heads in the first layers still averages and can be replaced by averaging, e.g. our proposed Gaussian weighting. In contrast, heads in the last layers steadily learn and seem to use metastable states to collect information created in lower layers. These heads seem to be a promising target for improving transformers. Neural networks with Hopfield networks outperform other methods on immune repertoire classification, where the Hopfield net stores several hundreds of thousands of patterns. We provide a new PyTorch layer called "Hopfield", which allows to equip deep learning architectures with modern Hopfield networks as a new powerful concept comprising pooling, memory, and attention. GitHub: https://github.com/ml-jku/hopfield-layers
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge