Víctor M. Campello
Federated learning in low-resource settings: A chest imaging study in Africa -- Challenges and lessons learned
May 20, 2025Abstract:This study explores the use of Federated Learning (FL) for tuberculosis (TB) diagnosis using chest X-rays in low-resource settings across Africa. FL allows hospitals to collaboratively train AI models without sharing raw patient data, addressing privacy concerns and data scarcity that hinder traditional centralized models. The research involved hospitals and research centers in eight African countries. Most sites used local datasets, while Ghana and The Gambia used public ones. The study compared locally trained models with a federated model built across all institutions to evaluate FL's real-world feasibility. Despite its promise, implementing FL in sub-Saharan Africa faces challenges such as poor infrastructure, unreliable internet, limited digital literacy, and weak AI regulations. Some institutions were also reluctant to share model updates due to data control concerns. In conclusion, FL shows strong potential for enabling AI-driven healthcare in underserved regions, but broader adoption will require improvements in infrastructure, education, and regulatory support.
In the Picture: Medical Imaging Datasets, Artifacts, and their Living Review
Jan 18, 2025


Abstract:Datasets play a critical role in medical imaging research, yet issues such as label quality, shortcuts, and metadata are often overlooked. This lack of attention may harm the generalizability of algorithms and, consequently, negatively impact patient outcomes. While existing medical imaging literature reviews mostly focus on machine learning (ML) methods, with only a few focusing on datasets for specific applications, these reviews remain static -- they are published once and not updated thereafter. This fails to account for emerging evidence, such as biases, shortcuts, and additional annotations that other researchers may contribute after the dataset is published. We refer to these newly discovered findings of datasets as research artifacts. To address this gap, we propose a living review that continuously tracks public datasets and their associated research artifacts across multiple medical imaging applications. Our approach includes a framework for the living review to monitor data documentation artifacts, and an SQL database to visualize the citation relationships between research artifact and dataset. Lastly, we discuss key considerations for creating medical imaging datasets, review best practices for data annotation, discuss the significance of shortcuts and demographic diversity, and emphasize the importance of managing datasets throughout their entire lifecycle. Our demo is publicly available at http://130.226.140.142.
PSFHS Challenge Report: Pubic Symphysis and Fetal Head Segmentation from Intrapartum Ultrasound Images
Sep 17, 2024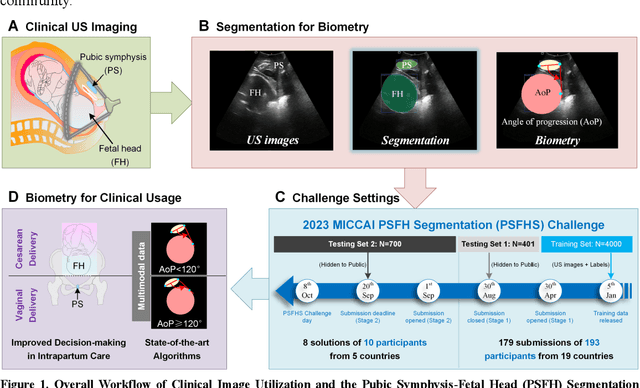
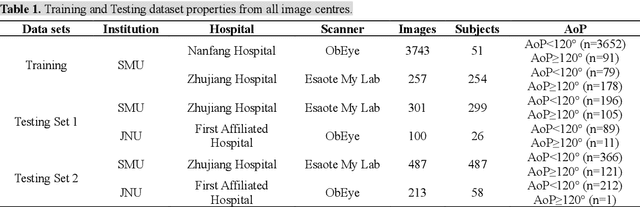
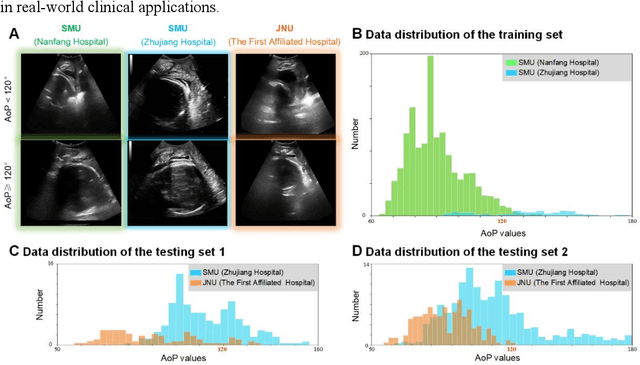

Abstract:Segmentation of the fetal and maternal structures, particularly intrapartum ultrasound imaging as advocated by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) for monitoring labor progression, is a crucial first step for quantitative diagnosis and clinical decision-making. This requires specialized analysis by obstetrics professionals, in a task that i) is highly time- and cost-consuming and ii) often yields inconsistent results. The utility of automatic segmentation algorithms for biometry has been proven, though existing results remain suboptimal. To push forward advancements in this area, the Grand Challenge on Pubic Symphysis-Fetal Head Segmentation (PSFHS) was held alongside the 26th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2023). This challenge aimed to enhance the development of automatic segmentation algorithms at an international scale, providing the largest dataset to date with 5,101 intrapartum ultrasound images collected from two ultrasound machines across three hospitals from two institutions. The scientific community's enthusiastic participation led to the selection of the top 8 out of 179 entries from 193 registrants in the initial phase to proceed to the competition's second stage. These algorithms have elevated the state-of-the-art in automatic PSFHS from intrapartum ultrasound images. A thorough analysis of the results pinpointed ongoing challenges in the field and outlined recommendations for future work. The top solutions and the complete dataset remain publicly available, fostering further advancements in automatic segmentation and biometry for intrapartum ultrasound imaging.
Democratizing AI in Africa: FL for Low-Resource Edge Devices
Aug 30, 2024
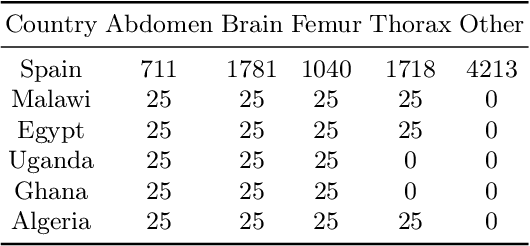
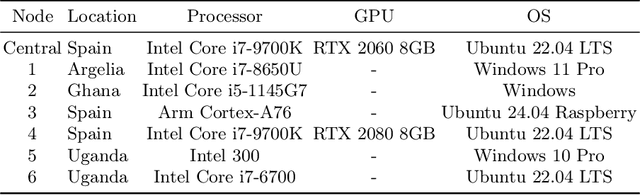
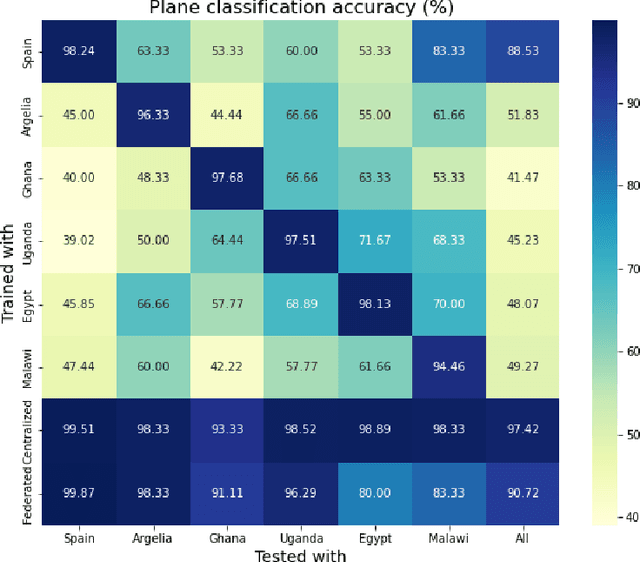
Abstract:Africa faces significant challenges in healthcare delivery due to limited infrastructure and access to advanced medical technologies. This study explores the use of federated learning to overcome these barriers, focusing on perinatal health. We trained a fetal plane classifier using perinatal data from five African countries: Algeria, Ghana, Egypt, Malawi, and Uganda, along with data from Spanish hospitals. To incorporate the lack of computational resources in the analysis, we considered a heterogeneous set of devices, including a Raspberry Pi and several laptops, for model training. We demonstrate comparative performance between a centralized and a federated model, despite the compute limitations, and a significant improvement in model generalizability when compared to models trained only locally. These results show the potential for a future implementation at a large scale of a federated learning platform to bridge the accessibility gap and improve model generalizability with very little requirements.
Generalisability of deep learning models in low-resource imaging settings: A fetal ultrasound study in 5 African countries
Sep 20, 2022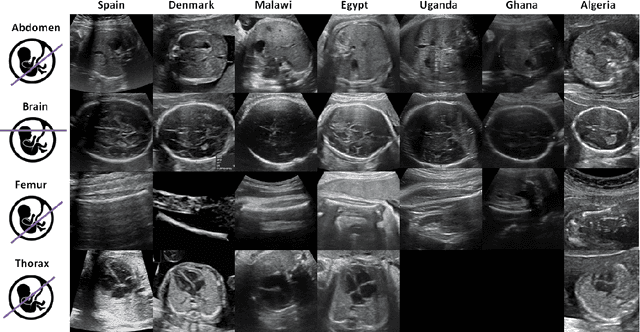
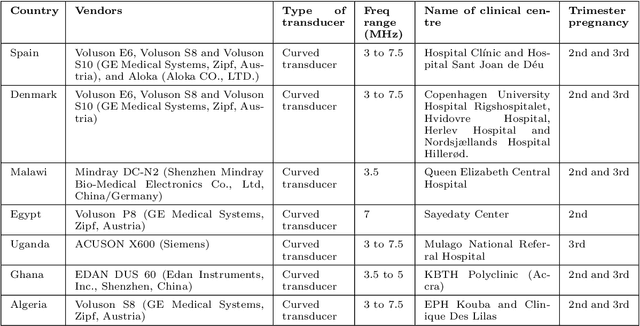
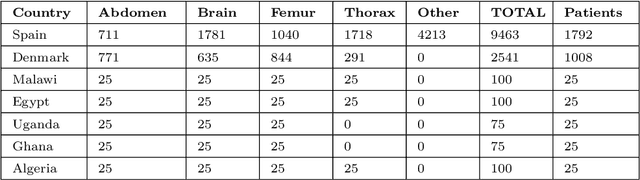
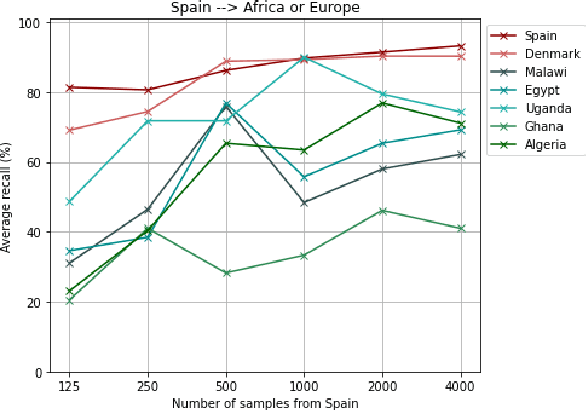
Abstract:Most artificial intelligence (AI) research have concentrated in high-income countries, where imaging data, IT infrastructures and clinical expertise are plentiful. However, slower progress has been made in limited-resource environments where medical imaging is needed. For example, in Sub-Saharan Africa the rate of perinatal mortality is very high due to limited access to antenatal screening. In these countries, AI models could be implemented to help clinicians acquire fetal ultrasound planes for diagnosis of fetal abnormalities. So far, deep learning models have been proposed to identify standard fetal planes, but there is no evidence of their ability to generalise in centres with limited access to high-end ultrasound equipment and data. This work investigates different strategies to reduce the domain-shift effect for a fetal plane classification model trained on a high-resource clinical centre and transferred to a new low-resource centre. To that end, a classifier trained with 1,792 patients from Spain is first evaluated on a new centre in Denmark in optimal conditions with 1,008 patients and is later optimised to reach the same performance in five African centres (Egypt, Algeria, Uganda, Ghana and Malawi) with 25 patients each. The results show that a transfer learning approach can be a solution to integrate small-size African samples with existing large-scale databases in developed countries. In particular, the model can be re-aligned and optimised to boost the performance on African populations by increasing the recall to $0.92 \pm 0.04$ and at the same time maintaining a high precision across centres. This framework shows promise for building new AI models generalisable across clinical centres with limited data acquired in challenging and heterogeneous conditions and calls for further research to develop new solutions for usability of AI in countries with less resources.
Multi-center, multi-vendor automated segmentation of left ventricular anatomy in contrast-enhanced MRI
Oct 28, 2021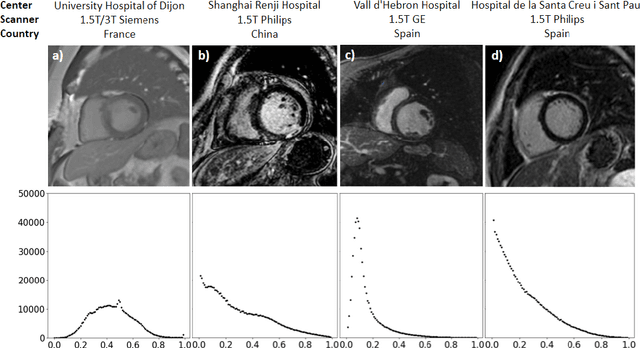
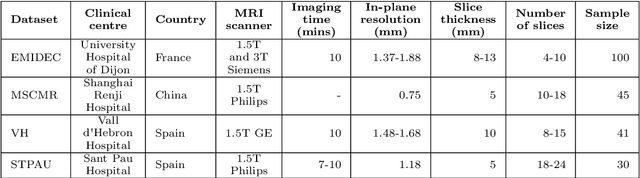
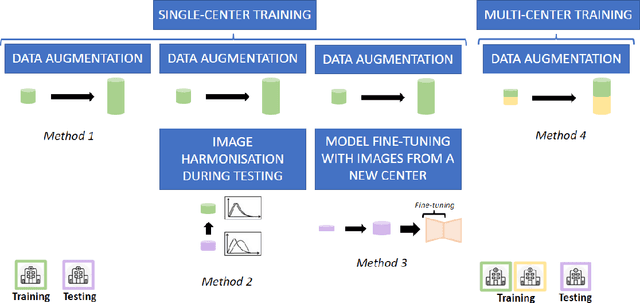
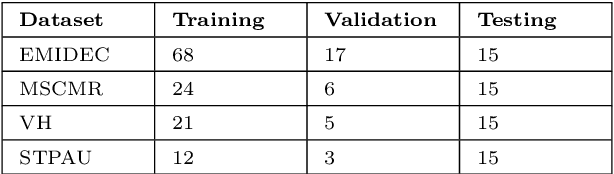
Abstract:Accurate delineation of the left ventricular boundaries in late gadolinium-enhanced magnetic resonance imaging (LGE-MRI) is an essential step for scar tissue quantification and patient-specific assessment of myocardial infarction. Many deep-learning techniques have been proposed to perform automatic segmentations of the left ventricle (LV) in LGE-MRI showing segmentations as accurate as those obtained by expert cardiologists. Thus far, the existing models have been overwhelmingly developed and evaluated with LGE-MRI datasets from single clinical centers. However, in practice, LGE-MRI images vary significantly between clinical centers within and across countries, in particular due to differences in the MRI scanners, imaging conditions, contrast injection protocols and local clinical practise. This work investigates for the first time multi-center and multi-vendor LV segmentation in LGE-MRI, by proposing, implementing and evaluating in detail several strategies to enhance model generalizability across clinical cites. These include data augmentation to artificially augment the image variability in the training sample, image harmonization to align the distributions of LGE-MRI images across centers, and transfer learning to adjust existing single-center models to unseen images from new clinical sites. The results obtained based on a new multi-center LGE-MRI dataset acquired in four clinical centers in Spain, France and China, show that the combination of data augmentation and transfer learning can lead to single-center models that generalize well to new clinical centers not included in the original training. The proposed framework shows the potential for developing clinical tools for automated LV segmentation in LGE-MRI that can be deployed in multiple clinical centers across distinct geographical locations.
Combining Multi-Sequence and Synthetic Images for Improved Segmentation of Late Gadolinium Enhancement Cardiac MRI
Sep 03, 2019



Abstract:Accurate segmentation of the cardiac boundaries in late gadolinium enhancement magnetic resonance images (LGE-MRI) is a fundamental step for accurate quantification of scar tissue. However, while there are many solutions for automatic cardiac segmentation of cine images, the presence of scar tissue can make the correct delineation of the myocardium in LGE-MRI challenging even for human experts. As part of the Multi-Sequence Cardiac MR Segmentation Challenge, we propose a solution for LGE-MRI segmentation based on two components. First, a generative adversarial network is trained for the task of modality-to-modality translation between cine and LGE-MRI sequences to obtain extra synthetic images for both modalities. Second, a deep learning model is trained for segmentation with different combinations of original, augmented and synthetic sequences. Our results based on three magnetic resonance sequences (LGE, bSSFP and T2) from 45 different patients show that the multi-sequence model training integrating synthetic images and data augmentation improves in the segmentation over conventional training with real datasets. In conclusion, the accuracy of the segmentation of LGE-MRI images can be improved by using complementary information provided by non-contrast MRI sequences.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge