Olivier Colliot
Sorbonne Université, Institut du Cerveau - Paris Brain Institute - ICM, CNRS, Inria, Inserm, AP-HP, Hôpital de la Pitié-Salpêtrière, F-75013, Paris, France
Performance uncertainty in medical image analysis: a large-scale investigation of confidence intervals
Jan 23, 2026Abstract:Performance uncertainty quantification is essential for reliable validation and eventual clinical translation of medical imaging artificial intelligence (AI). Confidence intervals (CIs) play a central role in this process by indicating how precise a reported performance estimate is. Yet, due to the limited amount of work examining CI behavior in medical imaging, the community remains largely unaware of how many diverse CI methods exist and how they behave in specific settings. The purpose of this study is to close this gap. To this end, we conducted a large-scale empirical analysis across a total of 24 segmentation and classification tasks, using 19 trained models per task group, a broad spectrum of commonly used performance metrics, multiple aggregation strategies, and several widely adopted CI methods. Reliability (coverage) and precision (width) of each CI method were estimated across all settings to characterize their dependence on study characteristics. Our analysis revealed five principal findings: 1) the sample size required for reliable CIs varies from a few dozens to several thousands of cases depending on study parameters; 2) CI behavior is strongly affected by the choice of performance metric; 3) aggregation strategy substantially influences the reliability of CIs, e.g. they require more observations for macro than for micro; 4) the machine learning problem (segmentation versus classification) modulates these effects; 5) different CI methods are not equally reliable and precise depending on the use case. These results form key components for the development of future guidelines on reporting performance uncertainty in medical imaging AI.
Medical Imaging AI Competitions Lack Fairness
Dec 19, 2025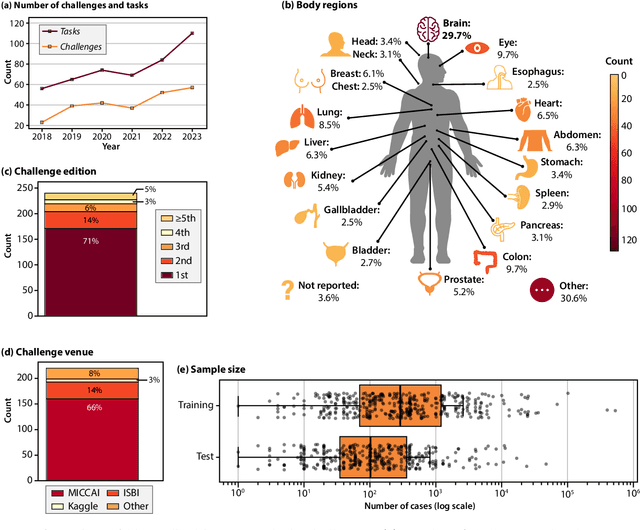
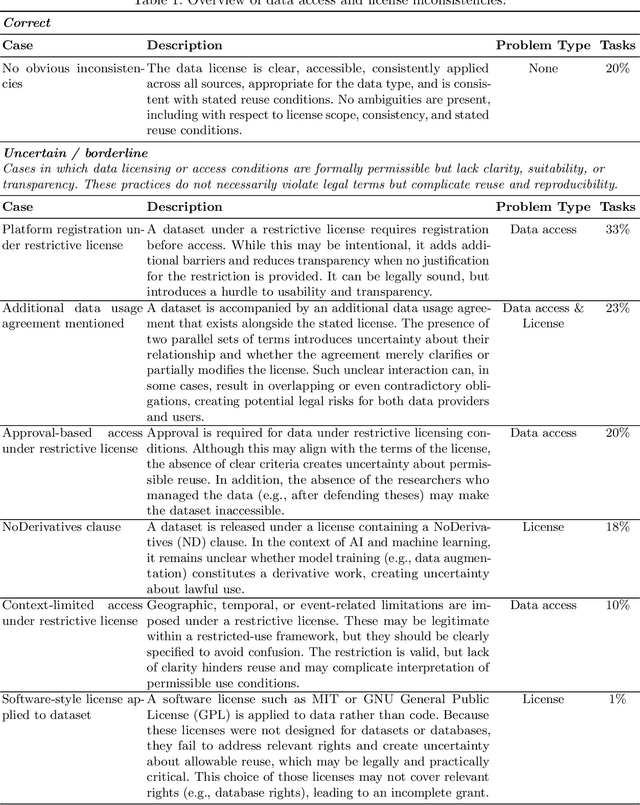
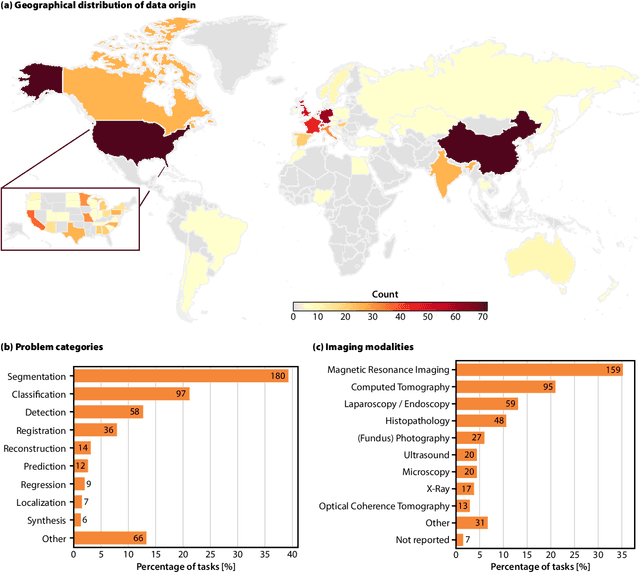
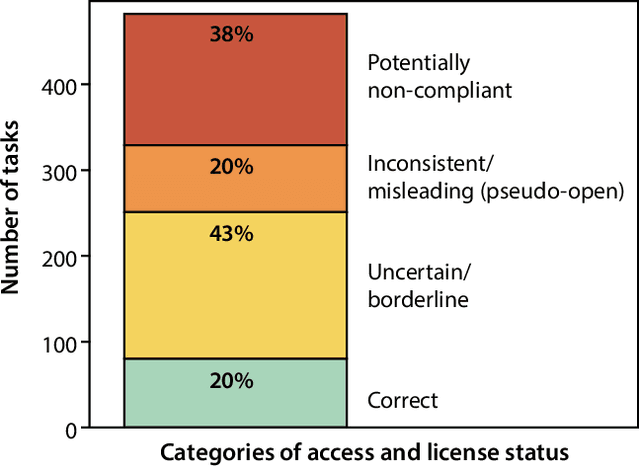
Abstract:Benchmarking competitions are central to the development of artificial intelligence (AI) in medical imaging, defining performance standards and shaping methodological progress. However, it remains unclear whether these benchmarks provide data that are sufficiently representative, accessible, and reusable to support clinically meaningful AI. In this work, we assess fairness along two complementary dimensions: (1) whether challenge datasets are representative of real-world clinical diversity, and (2) whether they are accessible and legally reusable in line with the FAIR principles. To address this question, we conducted a large-scale systematic study of 241 biomedical image analysis challenges comprising 458 tasks across 19 imaging modalities. Our findings show substantial biases in dataset composition, including geographic location, modality-, and problem type-related biases, indicating that current benchmarks do not adequately reflect real-world clinical diversity. Despite their widespread influence, challenge datasets were frequently constrained by restrictive or ambiguous access conditions, inconsistent or non-compliant licensing practices, and incomplete documentation, limiting reproducibility and long-term reuse. Together, these shortcomings expose foundational fairness limitations in our benchmarking ecosystem and highlight a disconnect between leaderboard success and clinical relevance.
False Promises in Medical Imaging AI? Assessing Validity of Outperformance Claims
May 07, 2025Abstract:Performance comparisons are fundamental in medical imaging Artificial Intelligence (AI) research, often driving claims of superiority based on relative improvements in common performance metrics. However, such claims frequently rely solely on empirical mean performance. In this paper, we investigate whether newly proposed methods genuinely outperform the state of the art by analyzing a representative cohort of medical imaging papers. We quantify the probability of false claims based on a Bayesian approach that leverages reported results alongside empirically estimated model congruence to estimate whether the relative ranking of methods is likely to have occurred by chance. According to our results, the majority (>80%) of papers claims outperformance when introducing a new method. Our analysis further revealed a high probability (>5%) of false outperformance claims in 86% of classification papers and 53% of segmentation papers. These findings highlight a critical flaw in current benchmarking practices: claims of outperformance in medical imaging AI are frequently unsubstantiated, posing a risk of misdirecting future research efforts.
SMILE-UHURA Challenge -- Small Vessel Segmentation at Mesoscopic Scale from Ultra-High Resolution 7T Magnetic Resonance Angiograms
Nov 14, 2024Abstract:The human brain receives nutrients and oxygen through an intricate network of blood vessels. Pathology affecting small vessels, at the mesoscopic scale, represents a critical vulnerability within the cerebral blood supply and can lead to severe conditions, such as Cerebral Small Vessel Diseases. The advent of 7 Tesla MRI systems has enabled the acquisition of higher spatial resolution images, making it possible to visualise such vessels in the brain. However, the lack of publicly available annotated datasets has impeded the development of robust, machine learning-driven segmentation algorithms. To address this, the SMILE-UHURA challenge was organised. This challenge, held in conjunction with the ISBI 2023, in Cartagena de Indias, Colombia, aimed to provide a platform for researchers working on related topics. The SMILE-UHURA challenge addresses the gap in publicly available annotated datasets by providing an annotated dataset of Time-of-Flight angiography acquired with 7T MRI. This dataset was created through a combination of automated pre-segmentation and extensive manual refinement. In this manuscript, sixteen submitted methods and two baseline methods are compared both quantitatively and qualitatively on two different datasets: held-out test MRAs from the same dataset as the training data (with labels kept secret) and a separate 7T ToF MRA dataset where both input volumes and labels are kept secret. The results demonstrate that most of the submitted deep learning methods, trained on the provided training dataset, achieved reliable segmentation performance. Dice scores reached up to 0.838 $\pm$ 0.066 and 0.716 $\pm$ 0.125 on the respective datasets, with an average performance of up to 0.804 $\pm$ 0.15.
Confidence intervals uncovered: Are we ready for real-world medical imaging AI?
Sep 27, 2024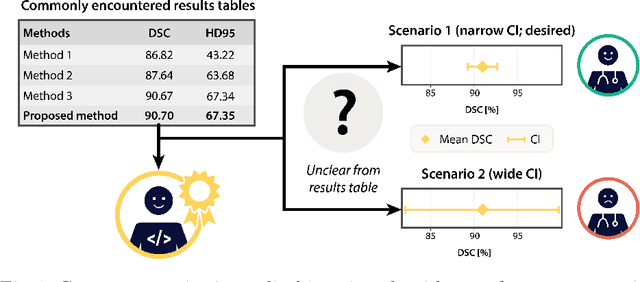
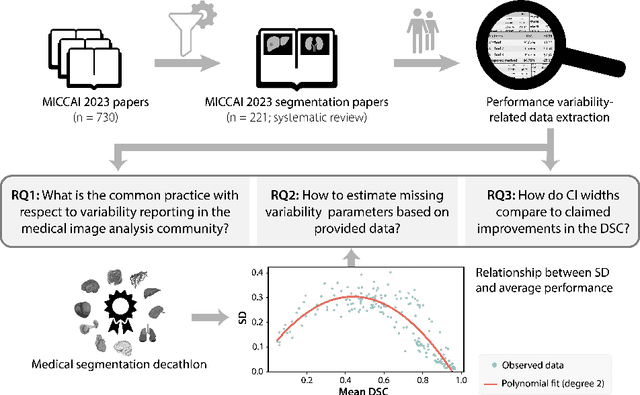
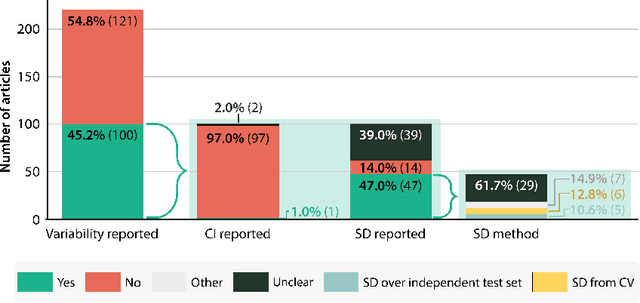

Abstract:Medical imaging is spearheading the AI transformation of healthcare. Performance reporting is key to determine which methods should be translated into clinical practice. Frequently, broad conclusions are simply derived from mean performance values. In this paper, we argue that this common practice is often a misleading simplification as it ignores performance variability. Our contribution is threefold. (1) Analyzing all MICCAI segmentation papers (n = 221) published in 2023, we first observe that more than 50% of papers do not assess performance variability at all. Moreover, only one (0.5%) paper reported confidence intervals (CIs) for model performance. (2) To address the reporting bottleneck, we show that the unreported standard deviation (SD) in segmentation papers can be approximated by a second-order polynomial function of the mean Dice similarity coefficient (DSC). Based on external validation data from 56 previous MICCAI challenges, we demonstrate that this approximation can accurately reconstruct the CI of a method using information provided in publications. (3) Finally, we reconstructed 95% CIs around the mean DSC of MICCAI 2023 segmentation papers. The median CI width was 0.03 which is three times larger than the median performance gap between the first and second ranked method. For more than 60% of papers, the mean performance of the second-ranked method was within the CI of the first-ranked method. We conclude that current publications typically do not provide sufficient evidence to support which models could potentially be translated into clinical practice.
Automatic rating of incomplete hippocampal inversions evaluated across multiple cohorts
Aug 05, 2024



Abstract:Incomplete Hippocampal Inversion (IHI), sometimes called hippocampal malrotation, is an atypical anatomical pattern of the hippocampus found in about 20% of the general population. IHI can be visually assessed on coronal slices of T1 weighted MR images, using a composite score that combines four anatomical criteria. IHI has been associated with several brain disorders (epilepsy, schizophrenia). However, these studies were based on small samples. Furthermore, the factors (genetic or environmental) that contribute to the genesis of IHI are largely unknown. Large-scale studies are thus needed to further understand IHI and their potential relationships to neurological and psychiatric disorders. However, visual evaluation is long and tedious, justifying the need for an automatic method. In this paper, we propose, for the first time, to automatically rate IHI. We proceed by predicting four anatomical criteria, which are then summed up to form the IHI score, providing the advantage of an interpretable score. We provided an extensive experimental investigation of different machine learning methods and training strategies. We performed automatic rating using a variety of deep learning models (conv5-FC3, ResNet and SECNN) as well as a ridge regression. We studied the generalization of our models using different cohorts and performed multi-cohort learning. We relied on a large population of 2,008 participants from the IMAGEN study, 993 and 403 participants from the QTIM/QTAB studies as well as 985 subjects from the UKBiobank. We showed that deep learning models outperformed a ridge regression. We demonstrated that the performances of the conv5-FC3 network were at least as good as more complex networks while maintaining a low complexity and computation time. We showed that training on a single cohort may lack in variability while training on several cohorts improves generalization.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024:016
Automated MRI Quality Assessment of Brain T1-weighted MRI in Clinical Data Warehouses: A Transfer Learning Approach Relying on Artefact Simulation
Jun 18, 2024



Abstract:The emergence of clinical data warehouses (CDWs), which contain the medical data of millions of patients, has paved the way for vast data sharing for research. The quality of MRIs gathered in CDWs differs greatly from what is observed in research settings and reflects a certain clinical reality. Consequently, a significant proportion of these images turns out to be unusable due to their poor quality. Given the massive volume of MRIs contained in CDWs, the manual rating of image quality is impossible. Thus, it is necessary to develop an automated solution capable of effectively identifying corrupted images in CDWs. This study presents an innovative transfer learning method for automated quality control of 3D gradient echo T1-weighted brain MRIs within a CDW, leveraging artefact simulation. We first intentionally corrupt images from research datasets by inducing poorer contrast, adding noise and introducing motion artefacts. Subsequently, three artefact-specific models are pre-trained using these corrupted images to detect distinct types of artefacts. Finally, the models are generalised to routine clinical data through a transfer learning technique, utilising 3660 manually annotated images. The overall image quality is inferred from the results of the three models, each designed to detect a specific type of artefact. Our method was validated on an independent test set of 385 3D gradient echo T1-weighted MRIs. Our proposed approach achieved excellent results for the detection of bad quality MRIs, with a balanced accuracy of over 87%, surpassing our previous approach by 3.5 percent points. Additionally, we achieved a satisfactory balanced accuracy of 79% for the detection of moderate quality MRIs, outperforming our previous performance by 5 percent points. Our framework provides a valuable tool for exploiting the potential of MRIs in CDWs.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024:012
Evaluation of pseudo-healthy image reconstruction for anomaly detection with deep generative models: Application to brain FDG PET
Jan 29, 2024Abstract:Over the past years, pseudo-healthy reconstruction for unsupervised anomaly detection has gained in popularity. This approach has the great advantage of not requiring tedious pixel-wise data annotation and offers possibility to generalize to any kind of anomalies, including that corresponding to rare diseases. By training a deep generative model with only images from healthy subjects, the model will learn to reconstruct pseudo-healthy images. This pseudo-healthy reconstruction is then compared to the input to detect and localize anomalies. The evaluation of such methods often relies on a ground truth lesion mask that is available for test data, which may not exist depending on the application. We propose an evaluation procedure based on the simulation of realistic abnormal images to validate pseudo-healthy reconstruction methods when no ground truth is available. This allows us to extensively test generative models on different kinds of anomalies and measuring their performance using the pair of normal and abnormal images corresponding to the same subject. It can be used as a preliminary automatic step to validate the capacity of a generative model to reconstruct pseudo-healthy images, before a more advanced validation step that would require clinician's expertise. We apply this framework to the reconstruction of 3D brain FDG PET using a convolutional variational autoencoder with the aim to detect as early as possible the neurodegeneration markers that are specific to dementia such as Alzheimer's disease.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024:003
Frequency Disentangled Learning for Segmentation of Midbrain Structures from Quantitative Susceptibility Mapping Data
Feb 25, 2023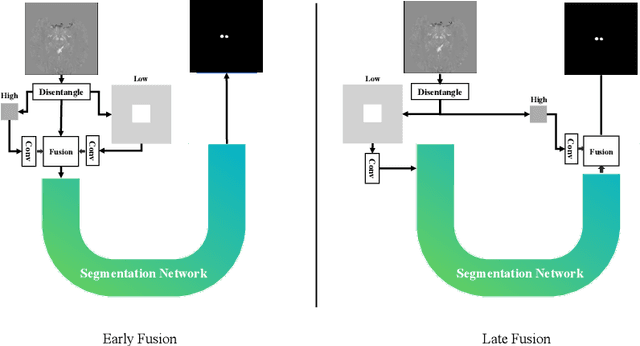

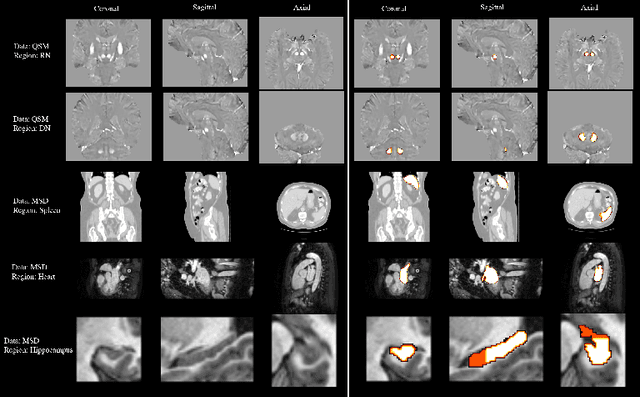
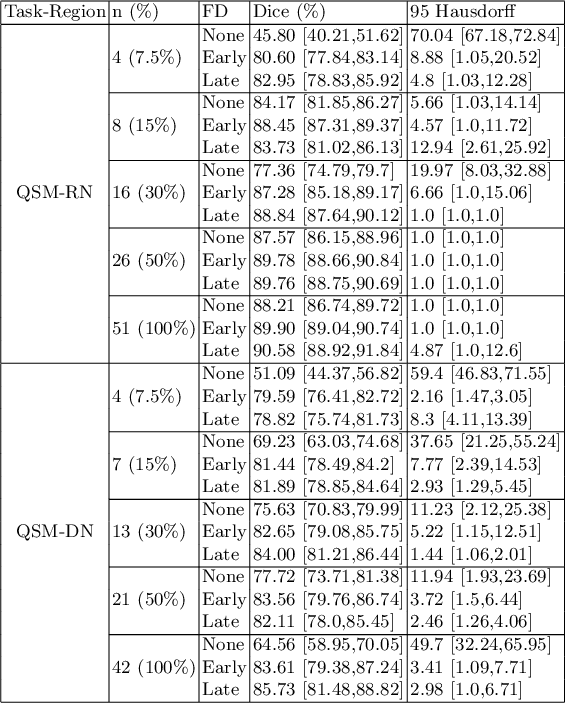
Abstract:One often lacks sufficient annotated samples for training deep segmentation models. This is in particular the case for less common imaging modalities such as Quantitative Susceptibility Mapping (QSM). It has been shown that deep models tend to fit the target function from low to high frequencies. One may hypothesize that such property can be leveraged for better training of deep learning models. In this paper, we exploit this property to propose a new training method based on frequency-domain disentanglement. It consists of two main steps: i) disentangling the image into high- and low-frequency parts and feature learning; ii) frequency-domain fusion to complete the task. The approach can be used with any backbone segmentation network. We apply the approach to the segmentation of the red and dentate nuclei from QSM data which is particularly relevant for the study of parkinsonian syndromes. We demonstrate that the proposed method provides considerable performance improvements for these tasks. We further applied it to three public datasets from the Medical Segmentation Decathlon (MSD) challenge. For two MSD tasks, it provided smaller but still substantial improvements (up to 7 points of Dice), especially under small training set situations.
Fourier Disentangled Multimodal Prior Knowledge Fusion for Red Nucleus Segmentation in Brain MRI
Nov 02, 2022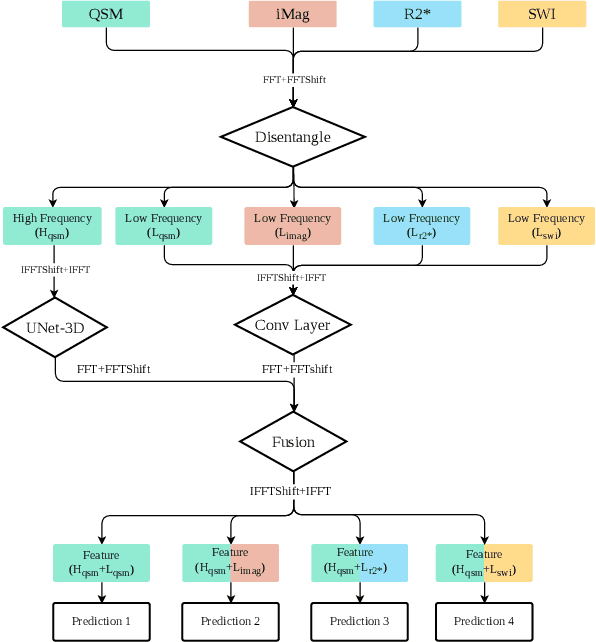
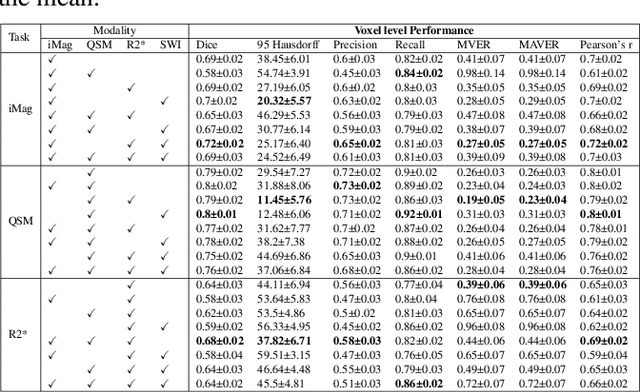
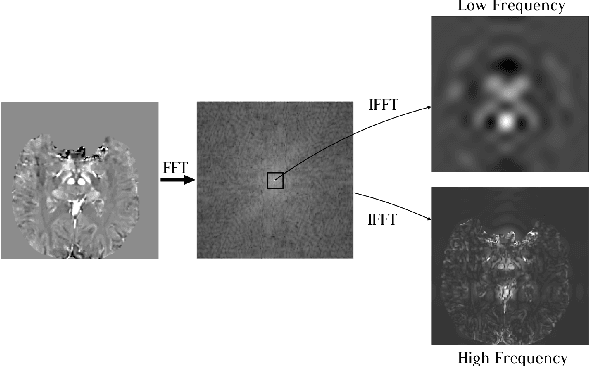
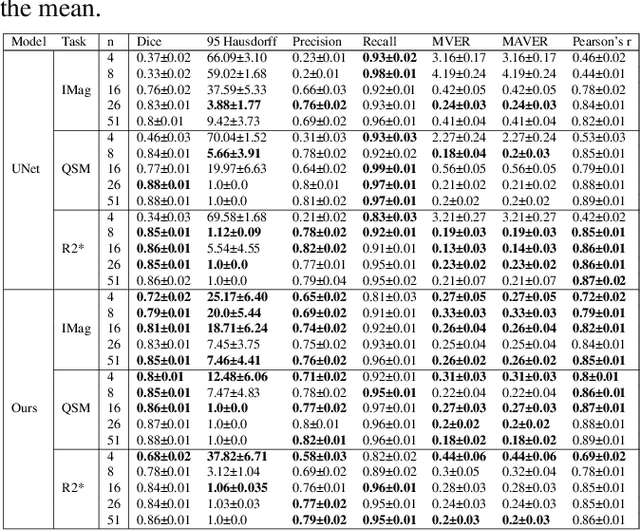
Abstract:Early and accurate diagnosis of parkinsonian syndromes is critical to provide appropriate care to patients and for inclusion in therapeutic trials. The red nucleus is a structure of the midbrain that plays an important role in these disorders. It can be visualized using iron-sensitive magnetic resonance imaging (MRI) sequences. Different iron-sensitive contrasts can be produced with MRI. Combining such multimodal data has the potential to improve segmentation of the red nucleus. Current multimodal segmentation algorithms are computationally consuming, cannot deal with missing modalities and need annotations for all modalities. In this paper, we propose a new model that integrates prior knowledge from different contrasts for red nucleus segmentation. The method consists of three main stages. First, it disentangles the image into high-level information representing the brain structure, and low-frequency information representing the contrast. The high-frequency information is then fed into a network to learn anatomical features, while the list of multimodal low-frequency information is processed by another module. Finally, feature fusion is performed to complete the segmentation task. The proposed method was used with several iron-sensitive contrasts (iMag, QSM, R2*, SWI). Experiments demonstrate that our proposed model substantially outperforms a baseline UNet model when the training set size is very small.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge