Sebastian Ströer
for The Alzheimer's Disease Neuroimaging Initiative, APPRIMAGE Study Group
Automated MRI Quality Assessment of Brain T1-weighted MRI in Clinical Data Warehouses: A Transfer Learning Approach Relying on Artefact Simulation
Jun 18, 2024



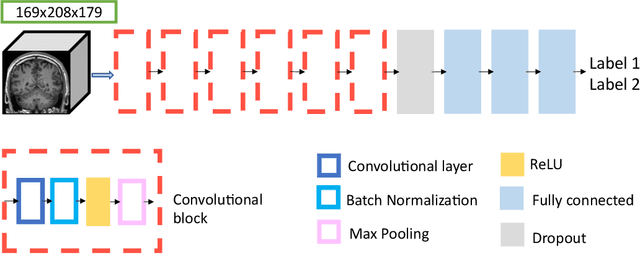
Abstract:The emergence of clinical data warehouses (CDWs), which contain the medical data of millions of patients, has paved the way for vast data sharing for research. The quality of MRIs gathered in CDWs differs greatly from what is observed in research settings and reflects a certain clinical reality. Consequently, a significant proportion of these images turns out to be unusable due to their poor quality. Given the massive volume of MRIs contained in CDWs, the manual rating of image quality is impossible. Thus, it is necessary to develop an automated solution capable of effectively identifying corrupted images in CDWs. This study presents an innovative transfer learning method for automated quality control of 3D gradient echo T1-weighted brain MRIs within a CDW, leveraging artefact simulation. We first intentionally corrupt images from research datasets by inducing poorer contrast, adding noise and introducing motion artefacts. Subsequently, three artefact-specific models are pre-trained using these corrupted images to detect distinct types of artefacts. Finally, the models are generalised to routine clinical data through a transfer learning technique, utilising 3660 manually annotated images. The overall image quality is inferred from the results of the three models, each designed to detect a specific type of artefact. Our method was validated on an independent test set of 385 3D gradient echo T1-weighted MRIs. Our proposed approach achieved excellent results for the detection of bad quality MRIs, with a balanced accuracy of over 87%, surpassing our previous approach by 3.5 percent points. Additionally, we achieved a satisfactory balanced accuracy of 79% for the detection of moderate quality MRIs, outperforming our previous performance by 5 percent points. Our framework provides a valuable tool for exploiting the potential of MRIs in CDWs.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024:012
Automatic quality control of brain T1-weighted magnetic resonance images for a clinical data warehouse
Apr 16, 2021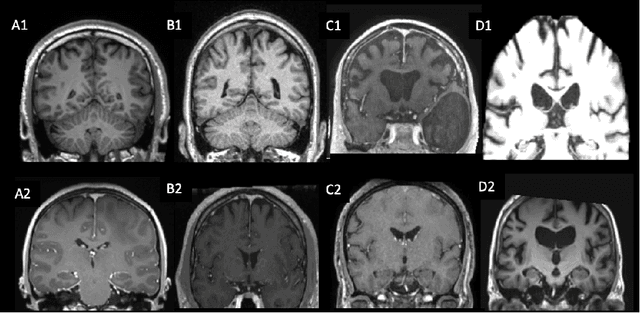
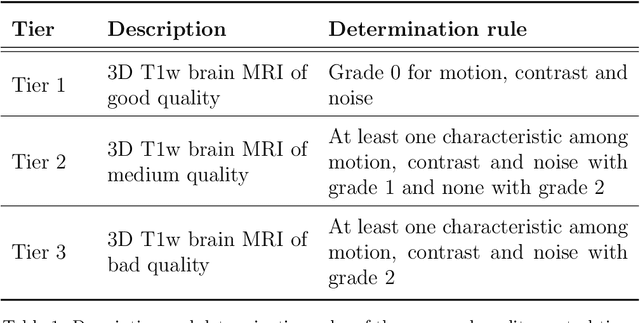
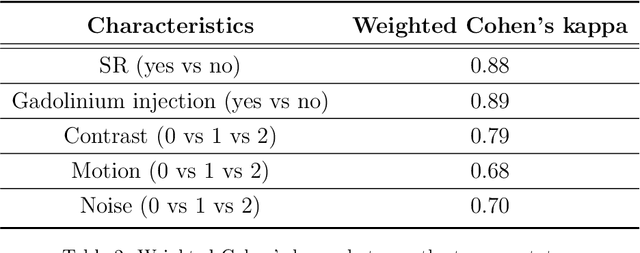
Abstract:Many studies on machine learning (ML) for computer-aided diagnosis have so far been mostly restricted to high-quality research data. Clinical data warehouses, gathering routine examinations from hospitals, offer great promises for training and validation of ML models in a realistic setting. However, the use of such clinical data warehouses requires quality control (QC) tools. Visual QC by experts is time-consuming and does not scale to large datasets. In this paper, we propose a convolutional neural network (CNN) for the automatic QC of 3D T1-weighted brain MRI for a large heterogeneous clinical data warehouse. To that purpose, we used the data warehouse of the hospitals of the Greater Paris area (Assistance Publique-H\^opitaux de Paris [AP-HP]). Specifically, the objectives were: 1) to identify images which are not proper T1-weighted brain MRIs; 2) to identify acquisitions for which gadolinium was injected; 3) to rate the overall image quality. We used 5000 images for training and validation and a separate set of 500 images for testing. In order to train/validate the CNN, the data were annotated by two trained raters according to a visual QC protocol that we specifically designed for application in the setting of a data warehouse. For objectives 1 and 2, our approach achieved excellent accuracy (balanced accuracy and F1-score \textgreater 90\%), similar to the human raters. For objective 3, the performance was good but substantially lower than that of human raters. Nevertheless, the automatic approach accurately identified (balanced accuracy and F1-score \textgreater 80\%) low quality images, which would typically need to be excluded. Overall, our approach shall be useful for exploiting hospital data warehouses in medical image computing.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge