Meliha Yetisgen
DermaVQA-DAS: Dermatology Assessment Schema (DAS) & Datasets for Closed-Ended Question Answering & Segmentation in Patient-Generated Dermatology Images
Dec 30, 2025Abstract:Recent advances in dermatological image analysis have been driven by large-scale annotated datasets; however, most existing benchmarks focus on dermatoscopic images and lack patient-authored queries and clinical context, limiting their applicability to patient-centered care. To address this gap, we introduce DermaVQA-DAS, an extension of the DermaVQA dataset that supports two complementary tasks: closed-ended question answering (QA) and dermatological lesion segmentation. Central to this work is the Dermatology Assessment Schema (DAS), a novel expert-developed framework that systematically captures clinically meaningful dermatological features in a structured and standardized form. DAS comprises 36 high-level and 27 fine-grained assessment questions, with multiple-choice options in English and Chinese. Leveraging DAS, we provide expert-annotated datasets for both closed QA and segmentation and benchmark state-of-the-art multimodal models. For segmentation, we evaluate multiple prompting strategies and show that prompt design impacts performance: the default prompt achieves the best results under Mean-of-Max and Mean-of-Mean evaluation aggregation schemes, while an augmented prompt incorporating both patient query title and content yields the highest performance under majority-vote-based microscore evaluation, achieving a Jaccard index of 0.395 and a Dice score of 0.566 with BiomedParse. For closed-ended QA, overall performance is strong across models, with average accuracies ranging from 0.729 to 0.798; o3 achieves the best overall accuracy (0.798), closely followed by GPT-4.1 (0.796), while Gemini-1.5-Pro shows competitive performance within the Gemini family (0.783). We publicly release DermaVQA-DAS, the DAS schema, and evaluation protocols to support and accelerate future research in patient-centered dermatological vision-language modeling (https://osf.io/72rp3).
Identifying Imaging Follow-Up in Radiology Reports: A Comparative Analysis of Traditional ML and LLM Approaches
Nov 14, 2025Abstract:Large language models (LLMs) have shown considerable promise in clinical natural language processing, yet few domain-specific datasets exist to rigorously evaluate their performance on radiology tasks. In this work, we introduce an annotated corpus of 6,393 radiology reports from 586 patients, each labeled for follow-up imaging status, to support the development and benchmarking of follow-up adherence detection systems. Using this corpus, we systematically compared traditional machine-learning classifiers, including logistic regression (LR), support vector machines (SVM), Longformer, and a fully fine-tuned Llama3-8B-Instruct, with recent generative LLMs. To evaluate generative LLMs, we tested GPT-4o and the open-source GPT-OSS-20B under two configurations: a baseline (Base) and a task-optimized (Advanced) setting that focused inputs on metadata, recommendation sentences, and their surrounding context. A refined prompt for GPT-OSS-20B further improved reasoning accuracy. Performance was assessed using precision, recall, and F1 scores with 95% confidence intervals estimated via non-parametric bootstrapping. Inter-annotator agreement was high (F1 = 0.846). GPT-4o (Advanced) achieved the best performance (F1 = 0.832), followed closely by GPT-OSS-20B (Advanced; F1 = 0.828). LR and SVM also performed strongly (F1 = 0.776 and 0.775), underscoring that while LLMs approach human-level agreement through prompt optimization, interpretable and resource-efficient models remain valuable baselines.
MORQA: Benchmarking Evaluation Metrics for Medical Open-Ended Question Answering
Sep 15, 2025



Abstract:Evaluating natural language generation (NLG) systems in the medical domain presents unique challenges due to the critical demands for accuracy, relevance, and domain-specific expertise. Traditional automatic evaluation metrics, such as BLEU, ROUGE, and BERTScore, often fall short in distinguishing between high-quality outputs, especially given the open-ended nature of medical question answering (QA) tasks where multiple valid responses may exist. In this work, we introduce MORQA (Medical Open-Response QA), a new multilingual benchmark designed to assess the effectiveness of NLG evaluation metrics across three medical visual and text-based QA datasets in English and Chinese. Unlike prior resources, our datasets feature 2-4+ gold-standard answers authored by medical professionals, along with expert human ratings for three English and Chinese subsets. We benchmark both traditional metrics and large language model (LLM)-based evaluators, such as GPT-4 and Gemini, finding that LLM-based approaches significantly outperform traditional metrics in correlating with expert judgments. We further analyze factors driving this improvement, including LLMs' sensitivity to semantic nuances and robustness to variability among reference answers. Our results provide the first comprehensive, multilingual qualitative study of NLG evaluation in the medical domain, highlighting the need for human-aligned evaluation methods. All datasets and annotations will be publicly released to support future research.
TrajSurv: Learning Continuous Latent Trajectories from Electronic Health Records for Trustworthy Survival Prediction
Aug 01, 2025Abstract:Trustworthy survival prediction is essential for clinical decision making. Longitudinal electronic health records (EHRs) provide a uniquely powerful opportunity for the prediction. However, it is challenging to accurately model the continuous clinical progression of patients underlying the irregularly sampled clinical features and to transparently link the progression to survival outcomes. To address these challenges, we develop TrajSurv, a model that learns continuous latent trajectories from longitudinal EHR data for trustworthy survival prediction. TrajSurv employs a neural controlled differential equation (NCDE) to extract continuous-time latent states from the irregularly sampled data, forming continuous latent trajectories. To ensure the latent trajectories reflect the clinical progression, TrajSurv aligns the latent state space with patient state space through a time-aware contrastive learning approach. To transparently link clinical progression to the survival outcome, TrajSurv uses latent trajectories in a two-step divide-and-conquer interpretation process. First, it explains how the changes in clinical features translate into the latent trajectory's evolution using a learned vector field. Second, it clusters these latent trajectories to identify key clinical progression patterns associated with different survival outcomes. Evaluations on two real-world medical datasets, MIMIC-III and eICU, show TrajSurv's competitive accuracy and superior transparency over existing deep learning methods.
A Scoping Review of Natural Language Processing in Addressing Medically Inaccurate Information: Errors, Misinformation, and Hallucination
Apr 16, 2025Abstract:Objective: This review aims to explore the potential and challenges of using Natural Language Processing (NLP) to detect, correct, and mitigate medically inaccurate information, including errors, misinformation, and hallucination. By unifying these concepts, the review emphasizes their shared methodological foundations and their distinct implications for healthcare. Our goal is to advance patient safety, improve public health communication, and support the development of more reliable and transparent NLP applications in healthcare. Methods: A scoping review was conducted following PRISMA guidelines, analyzing studies from 2020 to 2024 across five databases. Studies were selected based on their use of NLP to address medically inaccurate information and were categorized by topic, tasks, document types, datasets, models, and evaluation metrics. Results: NLP has shown potential in addressing medically inaccurate information on the following tasks: (1) error detection (2) error correction (3) misinformation detection (4) misinformation correction (5) hallucination detection (6) hallucination mitigation. However, challenges remain with data privacy, context dependency, and evaluation standards. Conclusion: This review highlights the advancements in applying NLP to tackle medically inaccurate information while underscoring the need to address persistent challenges. Future efforts should focus on developing real-world datasets, refining contextual methods, and improving hallucination management to ensure reliable and transparent healthcare applications.
Adapting Biomedical Abstracts into Plain language using Large Language Models
Jan 26, 2025



Abstract:A vast amount of medical knowledge is available for public use through online health forums, and question-answering platforms on social media. The majority of the population in the United States doesn't have the right amount of health literacy to make the best use of that information. Health literacy means the ability to obtain and comprehend the basic health information to make appropriate health decisions. To build the bridge between this gap, organizations advocate adapting this medical knowledge into plain language. Building robust systems to automate the adaptations helps both medical and non-medical professionals best leverage the available information online. The goal of the Plain Language Adaptation of Biomedical Abstracts (PLABA) track is to adapt the biomedical abstracts in English language extracted from PubMed based on the questions asked in MedlinePlus for the general public using plain language at the sentence level. As part of this track, we leveraged the best open-source Large Language Models suitable and fine-tuned for dialog use cases. We compare and present the results for all of our systems and our ranking among the other participants' submissions. Our top performing GPT-4 based model ranked first in the avg. simplicity measure and 3rd on the avg. accuracy measure.
MEDEC: A Benchmark for Medical Error Detection and Correction in Clinical Notes
Dec 26, 2024Abstract:Several studies showed that Large Language Models (LLMs) can answer medical questions correctly, even outperforming the average human score in some medical exams. However, to our knowledge, no study has been conducted to assess the ability of language models to validate existing or generated medical text for correctness and consistency. In this paper, we introduce MEDEC (https://github.com/abachaa/MEDEC), the first publicly available benchmark for medical error detection and correction in clinical notes, covering five types of errors (Diagnosis, Management, Treatment, Pharmacotherapy, and Causal Organism). MEDEC consists of 3,848 clinical texts, including 488 clinical notes from three US hospital systems that were not previously seen by any LLM. The dataset has been used for the MEDIQA-CORR shared task to evaluate seventeen participating systems [Ben Abacha et al., 2024]. In this paper, we describe the data creation methods and we evaluate recent LLMs (e.g., o1-preview, GPT-4, Claude 3.5 Sonnet, and Gemini 2.0 Flash) for the tasks of detecting and correcting medical errors requiring both medical knowledge and reasoning capabilities. We also conducted a comparative study where two medical doctors performed the same task on the MEDEC test set. The results showed that MEDEC is a sufficiently challenging benchmark to assess the ability of models to validate existing or generated notes and to correct medical errors. We also found that although recent LLMs have a good performance in error detection and correction, they are still outperformed by medical doctors in these tasks. We discuss the potential factors behind this gap, the insights from our experiments, the limitations of current evaluation metrics, and share potential pointers for future research.
Does Data Contamination Detection Work (Well) for LLMs? A Survey and Evaluation on Detection Assumptions
Oct 24, 2024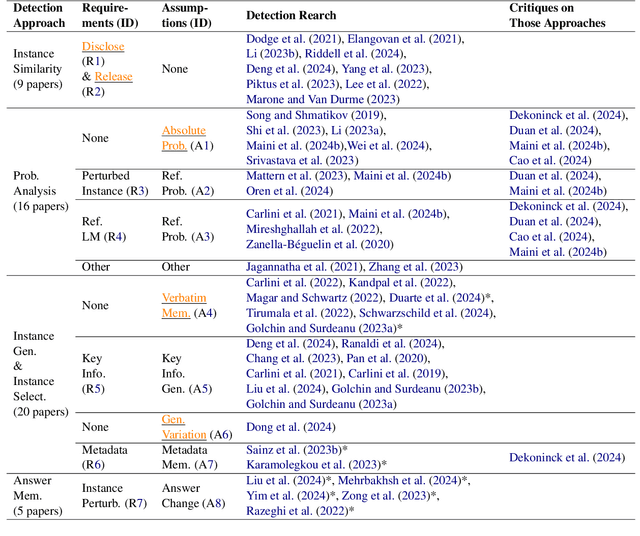
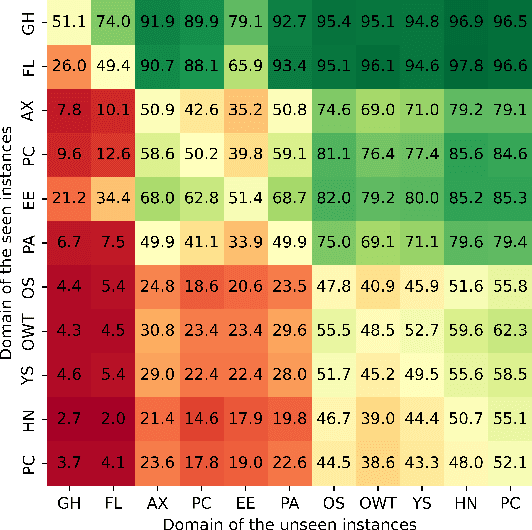
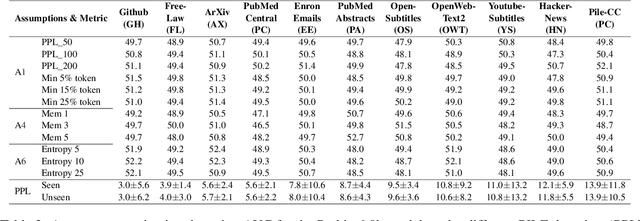
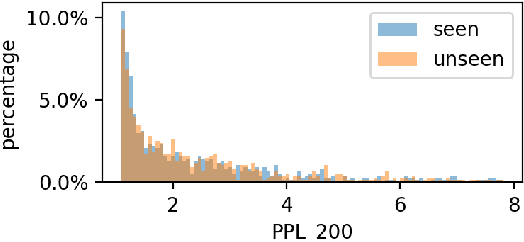
Abstract:Large language models (LLMs) have demonstrated great performance across various benchmarks, showing potential as general-purpose task solvers. However, as LLMs are typically trained on vast amounts of data, a significant concern in their evaluation is data contamination, where overlap between training data and evaluation datasets inflates performance assessments. While multiple approaches have been developed to identify data contamination, these approaches rely on specific assumptions that may not hold universally across different settings. To bridge this gap, we systematically review 47 papers on data contamination detection, categorize the underlying assumptions, and assess whether they have been rigorously validated. We identify and analyze eight categories of assumptions and test three of them as case studies. Our analysis reveals that when classifying instances used for pretraining LLMs, detection approaches based on these three assumptions perform close to random guessing, suggesting that current LLMs learn data distributions rather than memorizing individual instances. Overall, this work underscores the importance of approaches clearly stating their underlying assumptions and testing their validity across various scenarios.
BioMistral-NLU: Towards More Generalizable Medical Language Understanding through Instruction Tuning
Oct 24, 2024



Abstract:Large language models (LLMs) such as ChatGPT are fine-tuned on large and diverse instruction-following corpora, and can generalize to new tasks. However, those instruction-tuned LLMs often perform poorly in specialized medical natural language understanding (NLU) tasks that require domain knowledge, granular text comprehension, and structured data extraction. To bridge the gap, we: (1) propose a unified prompting format for 7 important NLU tasks, % through span extraction and multi-choice question-answering (QA), (2) curate an instruction-tuning dataset, MNLU-Instruct, utilizing diverse existing open-source medical NLU corpora, and (3) develop BioMistral-NLU, a generalizable medical NLU model, through fine-tuning BioMistral on MNLU-Instruct. We evaluate BioMistral-NLU in a zero-shot setting, across 6 important NLU tasks, from two widely adopted medical NLU benchmarks: Biomedical Language Understanding Evaluation (BLUE) and Biomedical Language Understanding and Reasoning Benchmark (BLURB). Our experiments show that our BioMistral-NLU outperforms the original BioMistral, as well as the proprietary LLMs - ChatGPT and GPT-4. Our dataset-agnostic prompting strategy and instruction tuning step over diverse NLU tasks enhance LLMs' generalizability across diverse medical NLU tasks. Our ablation experiments show that instruction-tuning on a wider variety of tasks, even when the total number of training instances remains constant, enhances downstream zero-shot generalization.
CACER: Clinical Concept Annotations for Cancer Events and Relations
Sep 05, 2024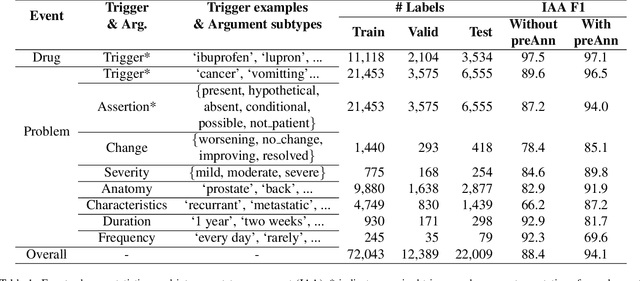
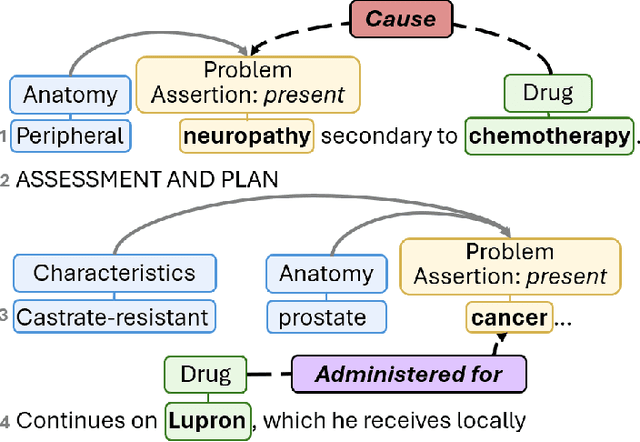
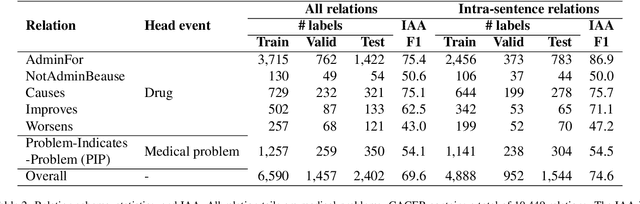
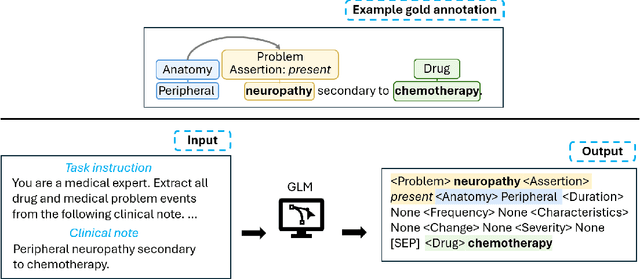
Abstract:Clinical notes contain unstructured representations of patient histories, including the relationships between medical problems and prescription drugs. To investigate the relationship between cancer drugs and their associated symptom burden, we extract structured, semantic representations of medical problem and drug information from the clinical narratives of oncology notes. We present Clinical Concept Annotations for Cancer Events and Relations (CACER), a novel corpus with fine-grained annotations for over 48,000 medical problems and drug events and 10,000 drug-problem and problem-problem relations. Leveraging CACER, we develop and evaluate transformer-based information extraction (IE) models such as BERT, Flan-T5, Llama3, and GPT-4 using fine-tuning and in-context learning (ICL). In event extraction, the fine-tuned BERT and Llama3 models achieved the highest performance at 88.2-88.0 F1, which is comparable to the inter-annotator agreement (IAA) of 88.4 F1. In relation extraction, the fine-tuned BERT, Flan-T5, and Llama3 achieved the highest performance at 61.8-65.3 F1. GPT-4 with ICL achieved the worst performance across both tasks. The fine-tuned models significantly outperformed GPT-4 in ICL, highlighting the importance of annotated training data and model optimization. Furthermore, the BERT models performed similarly to Llama3. For our task, LLMs offer no performance advantage over the smaller BERT models. The results emphasize the need for annotated training data to optimize models. Multiple fine-tuned transformer models achieved performance comparable to IAA for several extraction tasks.
* This is a pre-copy-editing, author-produced PDF of an article accepted for publication in JAMIA following peer review. The definitive publisher-authenticated version is available online at https://academic.oup.com/jamia/advance-article/doi/10.1093/jamia/ocae231/7748302
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge