Ijaz Gul
COVID-19: post infection implications in different age groups, mechanism, diagnosis, effective prevention, treatment, and recommendations
Jun 02, 2024



Abstract:SARS-CoV-2, the highly contagious pathogen responsible for the COVID-19 pandemic, has persistent effects that begin four weeks after initial infection and last for an undetermined duration. These chronic effects are more harmful than acute ones. This review explores the long-term impact of the virus on various human organs, including the pulmonary, cardiovascular, neurological, reproductive, gastrointestinal, musculoskeletal, endocrine, and lymphoid systems, particularly in older adults. Regarding diagnosis, RT-PCR is the gold standard for detecting COVID-19, though it requires specialized equipment, skilled personnel, and considerable time to produce results. To address these limitations, artificial intelligence in imaging and microfluidics technologies offers promising alternatives for diagnosing COVID-19 efficiently. Pharmacological and non-pharmacological strategies are effective in mitigating the persistent impacts of COVID-19. These strategies enhance immunity in post-COVID-19 patients by reducing cytokine release syndrome, improving T cell response, and increasing the circulation of activated natural killer and CD8 T cells in blood and tissues. This, in turn, alleviates symptoms such as fever, nausea, fatigue, muscle weakness, and pain. Vaccines, including inactivated viral, live attenuated viral, protein subunit, viral vectored, mRNA, DNA, and nanoparticle vaccines, significantly reduce the adverse long-term effects of the virus. However, no vaccine has been reported to provide lifetime protection against COVID-19. Consequently, protective measures such as physical distancing, mask usage, and hand hygiene remain essential strategies. This review offers a comprehensive understanding of the persistent effects of COVID-19 on individuals of varying ages, along with insights into diagnosis, treatment, vaccination, and future preventative measures against the spread of SARS-CoV-2.
Harnessing Intra-group Variations Via a Population-Level Context for Pathology Detection
Mar 04, 2024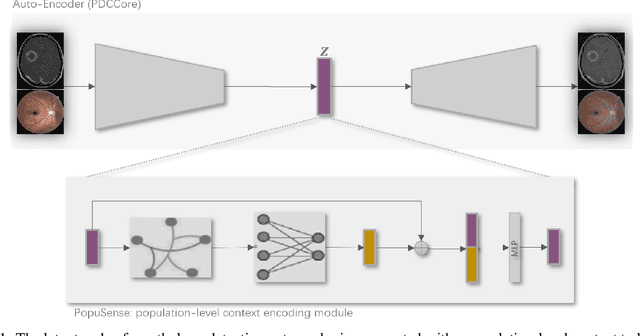
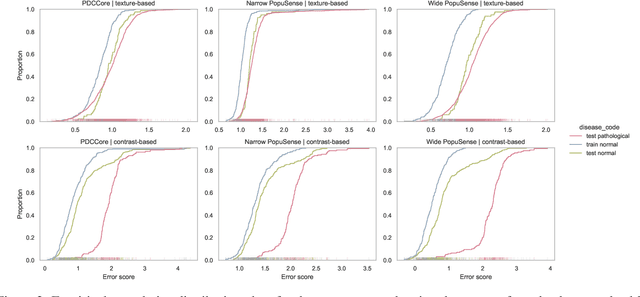
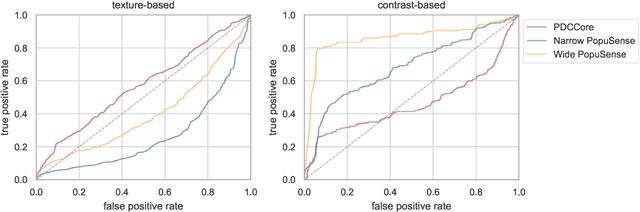
Abstract:Realizing sufficient separability between the distributions of healthy and pathological samples is a critical obstacle for pathology detection convolutional models. Moreover, these models exhibit a bias for contrast-based images, with diminished performance on texture-based medical images. This study introduces the notion of a population-level context for pathology detection and employs a graph theoretic approach to model and incorporate it into the latent code of an autoencoder via a refinement module we term PopuSense. PopuSense seeks to capture additional intra-group variations inherent in biomedical data that a local or global context of the convolutional model might miss or smooth out. Experiments on contrast-based and texture-based images, with minimal adaptation, encounter the existing preference for intensity-based input. Nevertheless, PopuSense demonstrates improved separability in contrast-based images, presenting an additional avenue for refining representations learned by a model.
Neuro-Symbolic Learning: Principles and Applications in Ophthalmology
Jul 31, 2022
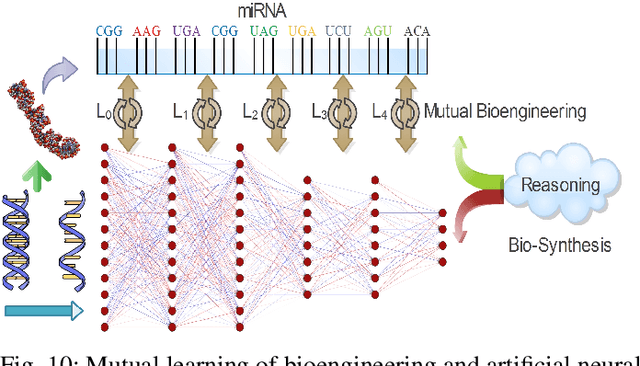
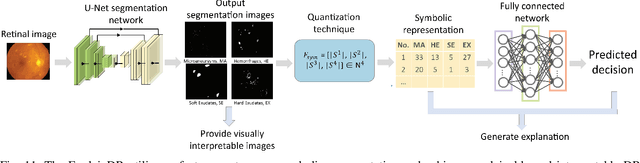
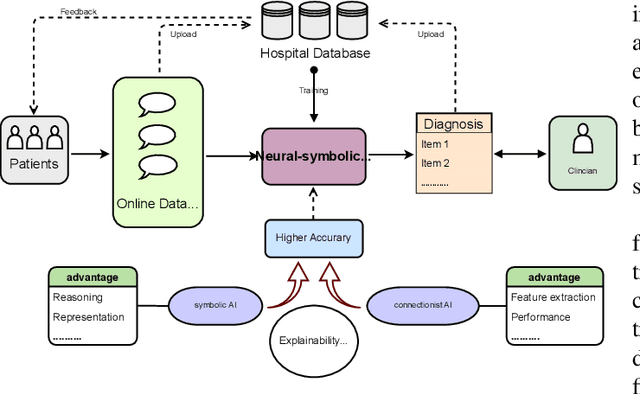
Abstract:Neural networks have been rapidly expanding in recent years, with novel strategies and applications. However, challenges such as interpretability, explainability, robustness, safety, trust, and sensibility remain unsolved in neural network technologies, despite the fact that they will unavoidably be addressed for critical applications. Attempts have been made to overcome the challenges in neural network computing by representing and embedding domain knowledge in terms of symbolic representations. Thus, the neuro-symbolic learning (NeSyL) notion emerged, which incorporates aspects of symbolic representation and bringing common sense into neural networks (NeSyL). In domains where interpretability, reasoning, and explainability are crucial, such as video and image captioning, question-answering and reasoning, health informatics, and genomics, NeSyL has shown promising outcomes. This review presents a comprehensive survey on the state-of-the-art NeSyL approaches, their principles, advances in machine and deep learning algorithms, applications such as opthalmology, and most importantly, future perspectives of this emerging field.
RCMNet: A deep learning model assists CAR-T therapy for leukemia
May 06, 2022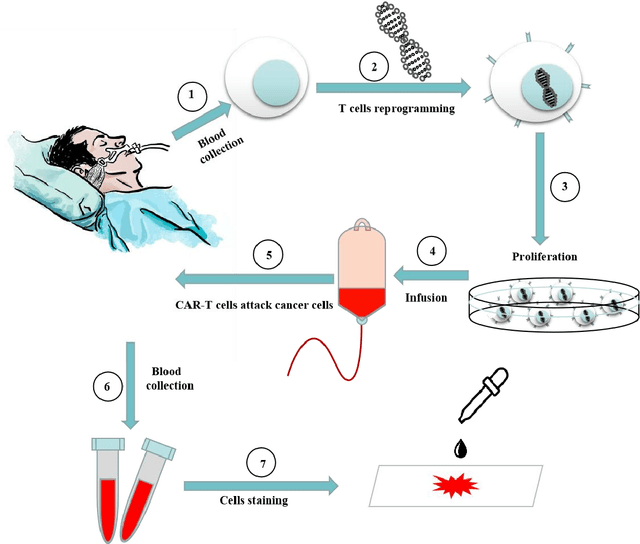
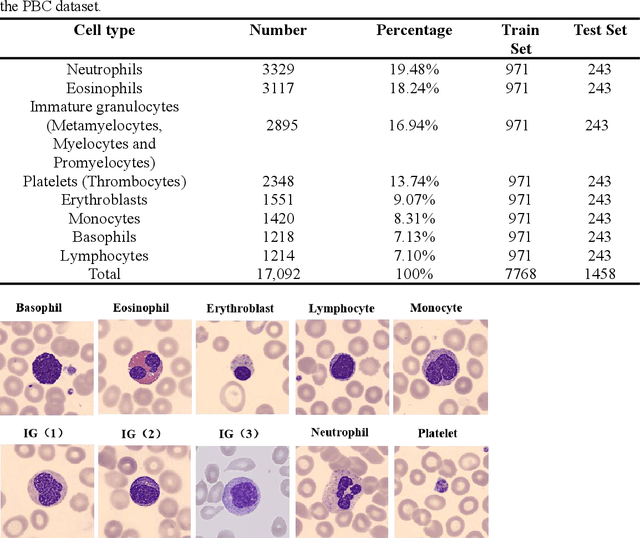
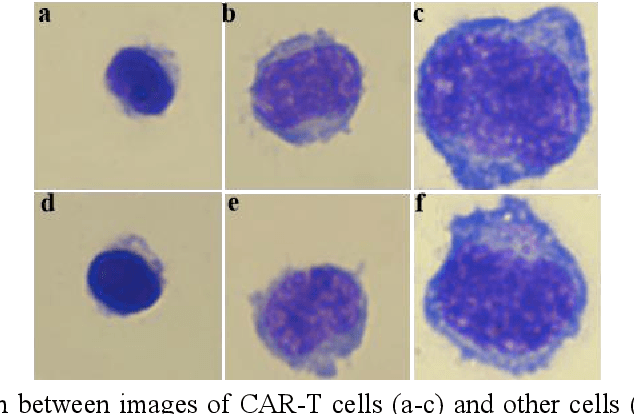

Abstract:Acute leukemia is a type of blood cancer with a high mortality rate. Current therapeutic methods include bone marrow transplantation, supportive therapy, and chemotherapy. Although a satisfactory remission of the disease can be achieved, the risk of recurrence is still high. Therefore, novel treatments are demanding. Chimeric antigen receptor-T (CAR-T) therapy has emerged as a promising approach to treat and cure acute leukemia. To harness the therapeutic potential of CAR-T cell therapy for blood diseases, reliable cell morphological identification is crucial. Nevertheless, the identification of CAR-T cells is a big challenge posed by their phenotypic similarity with other blood cells. To address this substantial clinical challenge, herein we first construct a CAR-T dataset with 500 original microscopy images after staining. Following that, we create a novel integrated model called RCMNet (ResNet18 with CBAM and MHSA) that combines the convolutional neural network (CNN) and Transformer. The model shows 99.63% top-1 accuracy on the public dataset. Compared with previous reports, our model obtains satisfactory results for image classification. Although testing on the CAR-T cells dataset, a decent performance is observed, which is attributed to the limited size of the dataset. Transfer learning is adapted for RCMNet and a maximum of 83.36% accuracy has been achieved, which is higher than other SOTA models. The study evaluates the effectiveness of RCMNet on a big public dataset and translates it to a clinical dataset for diagnostic applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge