Farhan Akram
Breast Cancer Immunohistochemical Image Generation: a Benchmark Dataset and Challenge Review
May 05, 2023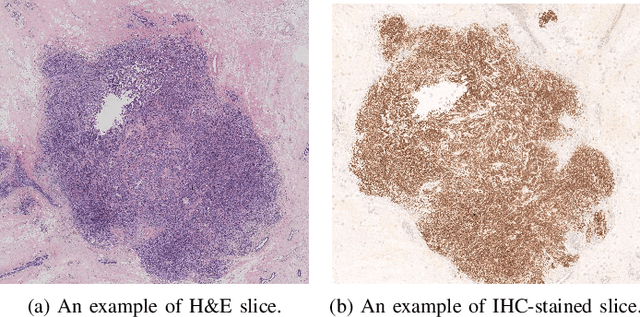
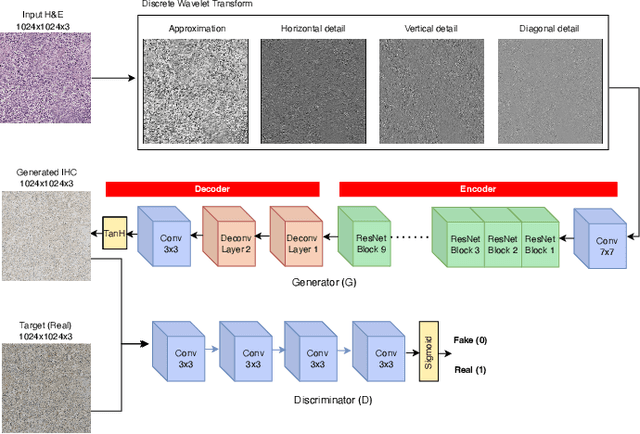
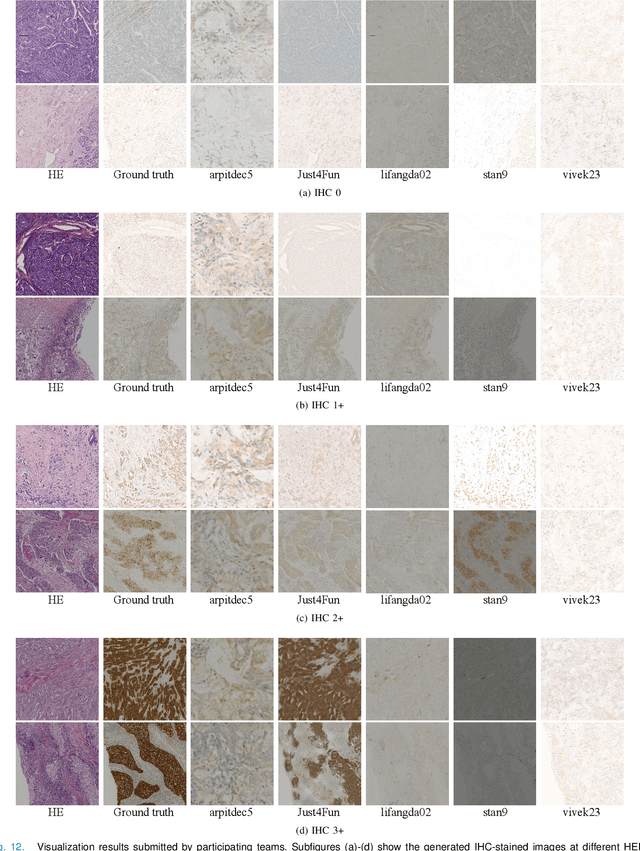
Abstract:For invasive breast cancer, immunohistochemical (IHC) techniques are often used to detect the expression level of human epidermal growth factor receptor-2 (HER2) in breast tissue to formulate a precise treatment plan. From the perspective of saving manpower, material and time costs, directly generating IHC-stained images from hematoxylin and eosin (H&E) stained images is a valuable research direction. Therefore, we held the breast cancer immunohistochemical image generation challenge, aiming to explore novel ideas of deep learning technology in pathological image generation and promote research in this field. The challenge provided registered H&E and IHC-stained image pairs, and participants were required to use these images to train a model that can directly generate IHC-stained images from corresponding H&E-stained images. We selected and reviewed the five highest-ranking methods based on their PSNR and SSIM metrics, while also providing overviews of the corresponding pipelines and implementations. In this paper, we further analyze the current limitations in the field of breast cancer immunohistochemical image generation and forecast the future development of this field. We hope that the released dataset and the challenge will inspire more scholars to jointly study higher-quality IHC-stained image generation.
Adversarial Learning with Multiscale Features and Kernel Factorization for Retinal Blood Vessel Segmentation
Jul 05, 2019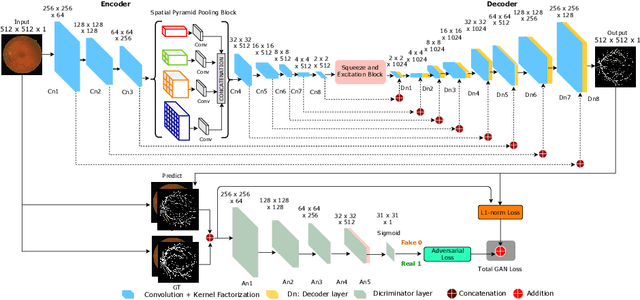
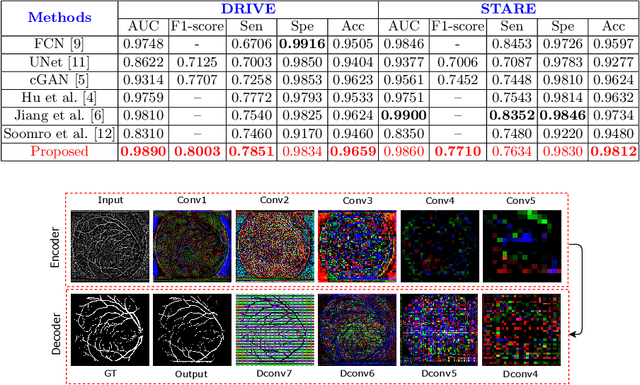
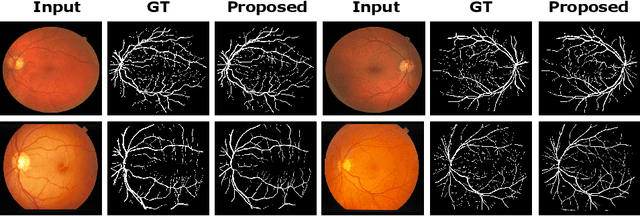
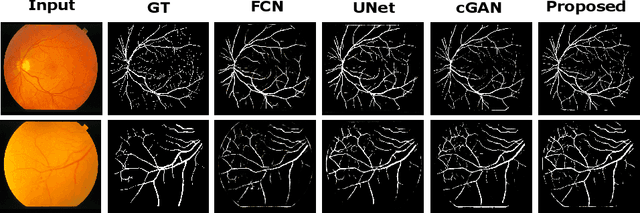
Abstract:In this paper, we propose an efficient blood vessel segmentation method for the eye fundus images using adversarial learning with multiscale features and kernel factorization. In the generator network of the adversarial framework, spatial pyramid pooling, kernel factorization and squeeze excitation block are employed to enhance the feature representation in spatial domain on different scales with reduced computational complexity. In turn, the discriminator network of the adversarial framework is formulated by combining convolutional layers with an additional squeeze excitation block to differentiate the generated segmentation mask from its respective ground truth. Before feeding the images to the network, we pre-processed them by using edge sharpening and Gaussian regularization to reach an optimized solution for vessel segmentation. The output of the trained model is post-processed using morphological operations to remove the small speckles of noise. The proposed method qualitatively and quantitatively outperforms state-of-the-art vessel segmentation methods using DRIVE and STARE datasets.
An Efficient Solution for Breast Tumor Segmentation and Classification in Ultrasound Images Using Deep Adversarial Learning
Jul 01, 2019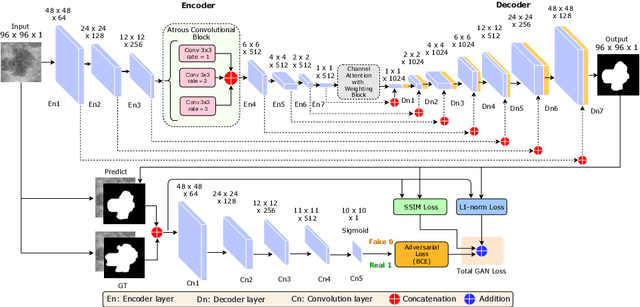
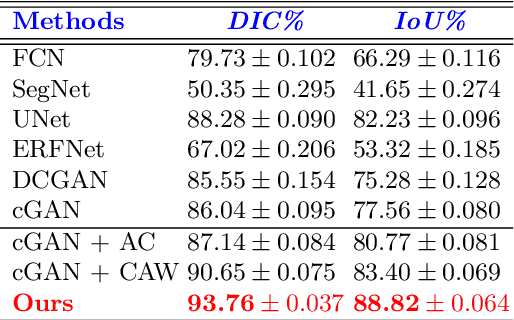
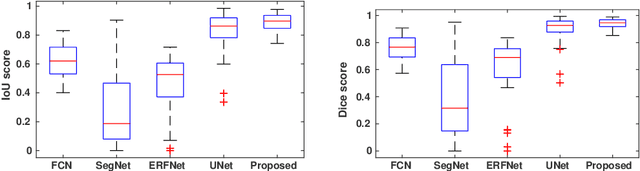
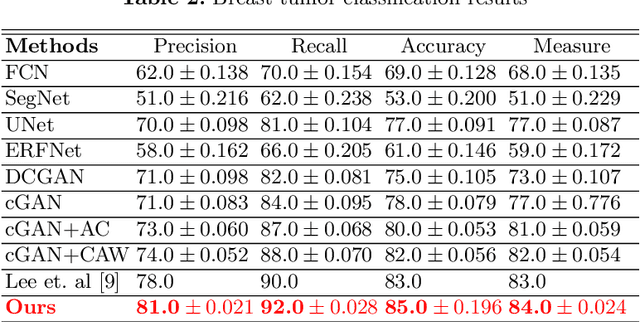
Abstract:This paper proposes an efficient solution for tumor segmentation and classification in breast ultrasound (BUS) images. We propose to add an atrous convolution layer to the conditional generative adversarial network (cGAN) segmentation model to learn tumor features at different resolutions of BUS images. To automatically re-balance the relative impact of each of the highest level encoded features, we also propose to add a channel-wise weighting block in the network. In addition, the SSIM and L1-norm loss with the typical adversarial loss are used as a loss function to train the model. Our model outperforms the state-of-the-art segmentation models in terms of the Dice and IoU metrics, achieving top scores of 93.76% and 88.82%, respectively. In the classification stage, we show that few statistics features extracted from the shape of the boundaries of the predicted masks can properly discriminate between benign and malignant tumors with an accuracy of 85%$
MobileGAN: Skin Lesion Segmentation Using a Lightweight Generative Adversarial Network
Jul 01, 2019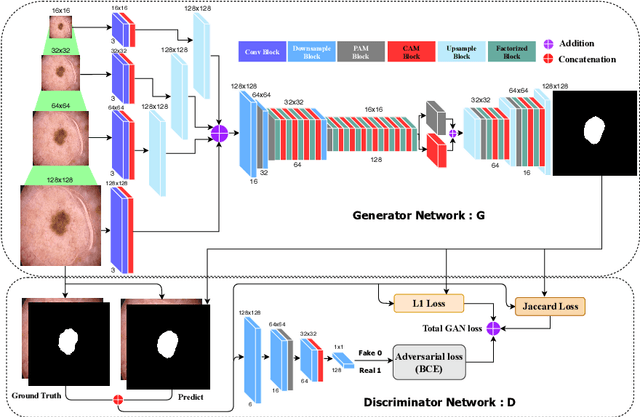
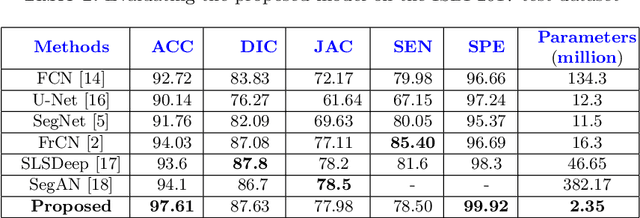
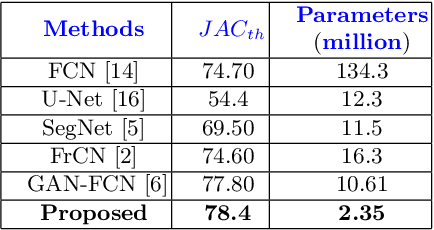
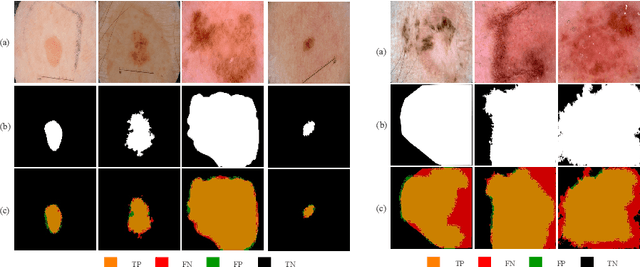
Abstract:Skin lesion segmentation in dermoscopic images is a challenge due to their blurry and irregular boundaries. Most of the segmentation approaches based on deep learning are time and memory consuming due to the hundreds of millions of parameters. Consequently, it is difficult to apply them to real dermatoscope devices with limited GPU and memory resources. In this paper, we propose a lightweight and efficient Generative Adversarial Networks (GAN) model, called MobileGAN for skin lesion segmentation. More precisely, the MobileGAN combines 1D non-bottleneck factorization networks with position and channel attention modules in a GAN model. The proposed model is evaluated on the test dataset of the ISBI 2017 challenges and the validation dataset of ISIC 2018 challenges. Although the proposed network has only 2.35 millions of parameters, it is still comparable with the state-of-the-art. The experimental results show that our MobileGAN obtains comparable performance with an accuracy of 97.61%.
Fence GAN: Towards Better Anomaly Detection
Apr 02, 2019



Abstract:Anomaly detection is a classical problem where the aim is to detect anomalous data that do not belong to the normal data distribution. Current state-of-the-art methods for anomaly detection on complex high-dimensional data are based on the generative adversarial network (GAN). However, the traditional GAN loss is not directly aligned with the anomaly detection objective: it encourages the distribution of the generated samples to overlap with the real data and so the resulting discriminator has been found to be ineffective as an anomaly detector. In this paper, we propose simple modifications to the GAN loss such that the generated samples lie at the boundary of the real data distribution. With our modified GAN loss, our anomaly detection method, called Fence GAN (FGAN), directly uses the discriminator score as an anomaly threshold. Our experimental results using the MNIST, CIFAR10 and KDD99 datasets show that Fence GAN yields the best anomaly classification accuracy compared to state-of-the-art methods.
Breast Tumor Segmentation and Shape Classification in Mammograms using Generative Adversarial and Convolutional Neural Network
Oct 23, 2018



Abstract:Mammogram inspection in search of breast tumors is a tough assignment that radiologists must carry out frequently. Therefore, image analysis methods are needed for the detection and delineation of breast masses, which portray crucial morphological information that will support reliable diagnosis. In this paper, we proposed a conditional Generative Adversarial Network (cGAN) devised to segment a breast mass within a region of interest (ROI) in a mammogram. The generative network learns to recognize the breast mass area and to create the binary mask that outlines the breast mass. In turn, the adversarial network learns to distinguish between real (ground truth) and synthetic segmentations, thus enforcing the generative network to create binary masks as realistic as possible. The cGAN works well even when the number of training samples are limited. Therefore, the proposed method outperforms several state-of-the-art approaches. This hypothesis is corroborated by diverse experiments performed on two datasets, the public INbreast and a private in-house dataset. The proposed segmentation model provides a high Dice coefficient and Intersection over Union (IoU) of 94% and 87%, respectively. In addition, a shape descriptor based on a Convolutional Neural Network (CNN) is proposed to classify the generated masks into four mass shapes: irregular, lobular, oval and round. The proposed shape descriptor was trained on Digital Database for Screening Mammography (DDSM) yielding an overall accuracy of 80%, which outperforms the current state-of-the-art.
REFUGE CHALLENGE 2018-Task 2:Deep Optic Disc and Cup Segmentation in Fundus Images Using U-Net and Multi-scale Feature Matching Networks
Jul 30, 2018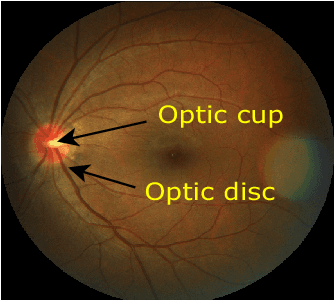

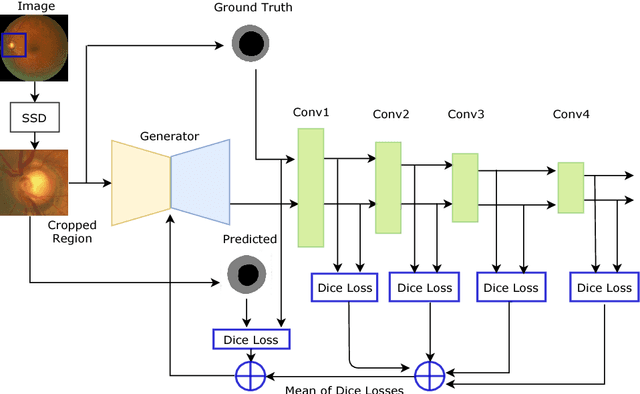
Abstract:In this paper, an optic disc and cup segmentation method is proposed using U-Net followed by a multi-scale feature matching network. The proposed method targets task 2 of the REFUGE challenge 2018. In order to solve the segmentation problem of task 2, we firstly crop the input image using single shot multibox detector (SSD). The cropped image is then passed to an encoder-decoder network with skip connections also known as generator. Afterwards, both the ground truth and generated images are fed to a convolution neural network (CNN) to extract their multi-level features. A dice loss function is then used to match the features of the two images by minimizing the error at each layer. The aggregation of error from each layer is back-propagated through the generator network to enforce it to generate a segmented image closer to the ground truth. The CNN network improves the performance of the generator network without increasing the complexity of the model.
Retinal Optic Disc Segmentation using Conditional Generative Adversarial Network
Jun 11, 2018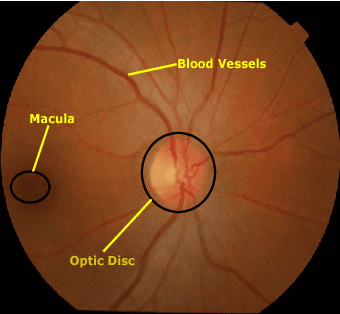
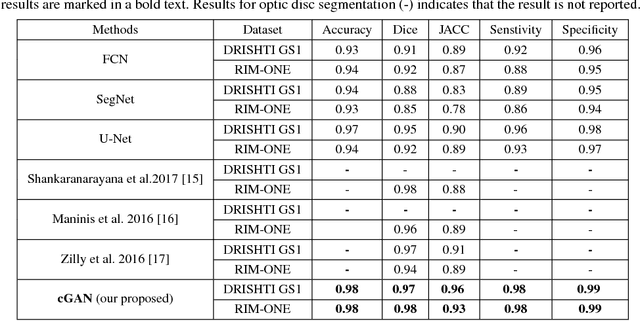
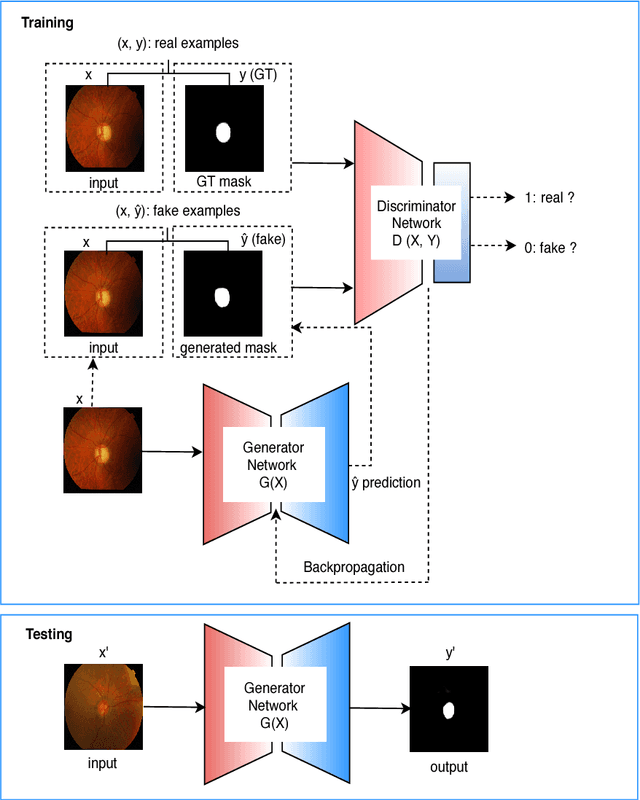

Abstract:This paper proposed a retinal image segmentation method based on conditional Generative Adversarial Network (cGAN) to segment optic disc. The proposed model consists of two successive networks: generator and discriminator. The generator learns to map information from the observing input (i.e., retinal fundus color image), to the output (i.e., binary mask). Then, the discriminator learns as a loss function to train this mapping by comparing the ground-truth and the predicted output with observing the input image as a condition.Experiments were performed on two publicly available dataset; DRISHTI GS1 and RIM-ONE. The proposed model outperformed state-of-the-art-methods by achieving around 0.96% and 0.98% of Jaccard and Dice coefficients, respectively. Moreover, an image segmentation is performed in less than a second on recent GPU.
Conditional Generative Adversarial and Convolutional Networks for X-ray Breast Mass Segmentation and Shape Classification
Jun 10, 2018


Abstract:This paper proposes a novel approach based on conditional Generative Adversarial Networks (cGAN) for breast mass segmentation in mammography. We hypothesized that the cGAN structure is well-suited to accurately outline the mass area, especially when the training data is limited. The generative network learns intrinsic features of tumors while the adversarial network enforces segmentations to be similar to the ground truth. Experiments performed on dozens of malignant tumors extracted from the public DDSM dataset and from our in-house private dataset confirm our hypothesis with very high Dice coefficient and Jaccard index (>94% and >89%, respectively) outperforming the scores obtained by other state-of-the-art approaches. Furthermore, in order to detect portray significant morphological features of the segmented tumor, a specific Convolutional Neural Network (CNN) have also been designed for classifying the segmented tumor areas into four types (irregular, lobular, oval and round), which provides an overall accuracy about 72% with the DDSM dataset.
SLSDeep: Skin Lesion Segmentation Based on Dilated Residual and Pyramid Pooling Networks
May 31, 2018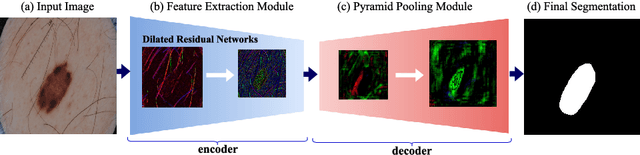
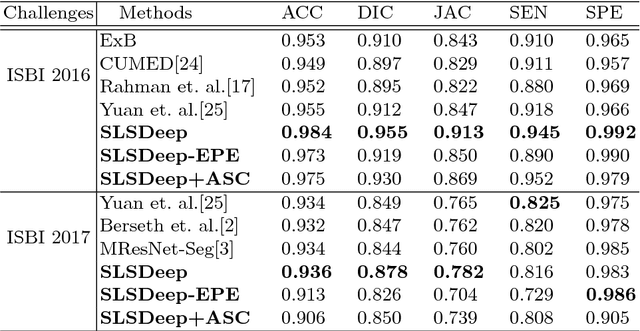
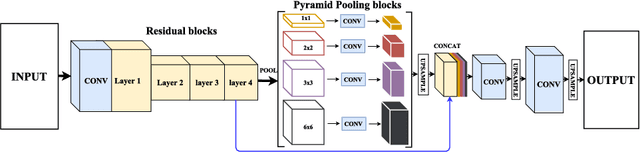
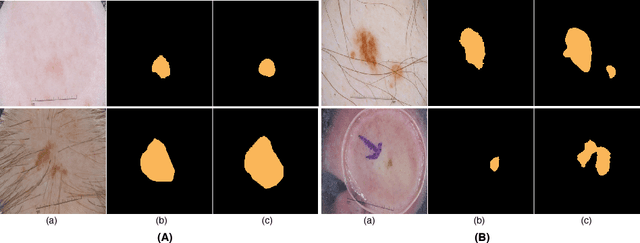
Abstract:Skin lesion segmentation (SLS) in dermoscopic images is a crucial task for automated diagnosis of melanoma. In this paper, we present a robust deep learning SLS model, so-called SLSDeep, which is represented as an encoder-decoder network. The encoder network is constructed by dilated residual layers, in turn, a pyramid pooling network followed by three convolution layers is used for the decoder. Unlike the traditional methods employing a cross-entropy loss, we investigated a loss function by combining both Negative Log Likelihood (NLL) and End Point Error (EPE) to accurately segment the melanoma regions with sharp boundaries. The robustness of the proposed model was evaluated on two public databases: ISBI 2016 and 2017 for skin lesion analysis towards melanoma detection challenge. The proposed model outperforms the state-of-the-art methods in terms of segmentation accuracy. Moreover, it is capable to segment more than $100$ images of size 384x384 per second on a recent GPU.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge