Daniel Grzech
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Uncertainty quantification in non-rigid image registration via stochastic gradient Markov chain Monte Carlo
Oct 25, 2021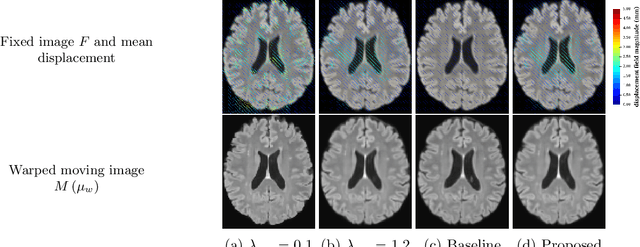
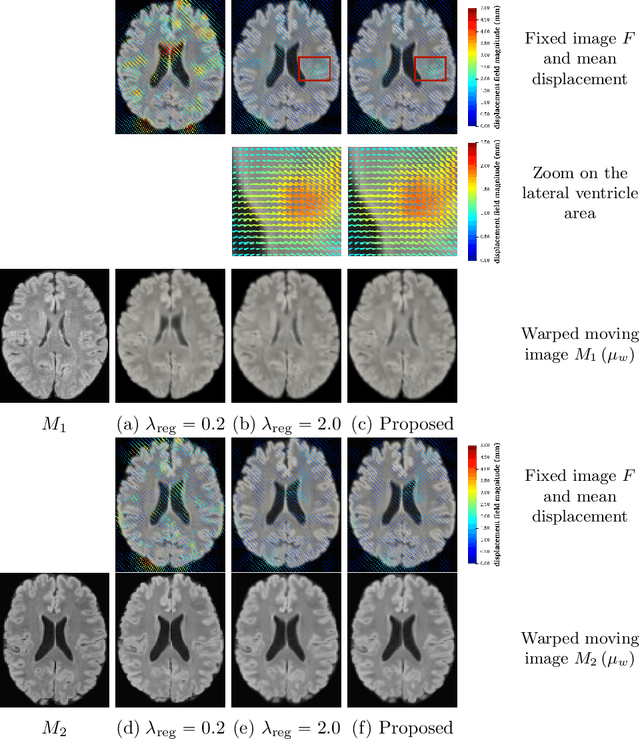
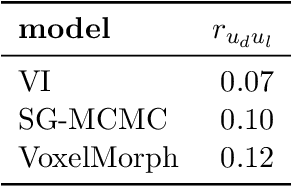
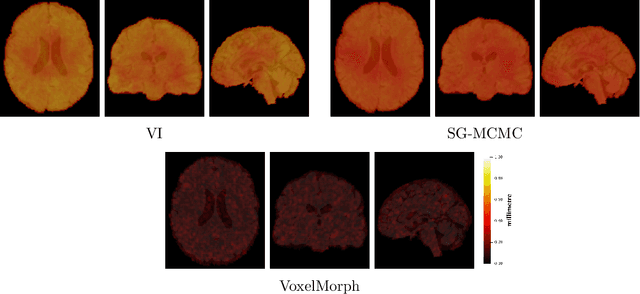
Abstract:We develop a new Bayesian model for non-rigid registration of three-dimensional medical images, with a focus on uncertainty quantification. Probabilistic registration of large images with calibrated uncertainty estimates is difficult for both computational and modelling reasons. To address the computational issues, we explore connections between the Markov chain Monte Carlo by backpropagation and the variational inference by backpropagation frameworks, in order to efficiently draw samples from the posterior distribution of transformation parameters. To address the modelling issues, we formulate a Bayesian model for image registration that overcomes the existing barriers when using a dense, high-dimensional, and diffeomorphic transformation parametrisation. This results in improved calibration of uncertainty estimates. We compare the model in terms of both image registration accuracy and uncertainty quantification to VoxelMorph, a state-of-the-art image registration model based on deep learning.
FastReg: Fast Non-Rigid Registration via Accelerated Optimisation on the Manifold of Diffeomorphisms
Mar 05, 2019



Abstract:We present a new approach to diffeomorphic non-rigid registration of medical images. The method is based on optical flow and warps images via gradient flow with the standard $L^2$ inner product. To compute the transformation, we rely on accelerated optimisation on the manifold of diffeomorphisms. We achieve regularity properties of Sobolev gradient flows, which are expensive to compute, owing to a novel method of averaging the gradients in time rather than space. We successfully register brain MRI and challenging abdominal CT scans at speeds orders of magnitude faster than previous approaches. We make our code available in a public repository: https://github.com/dgrzech/fastreg
EchoFusion: Tracking and Reconstruction of Objects in 4D Freehand Ultrasound Imaging without External Trackers
Jul 19, 2018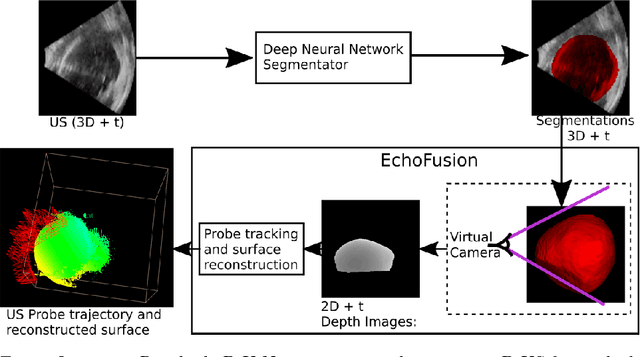

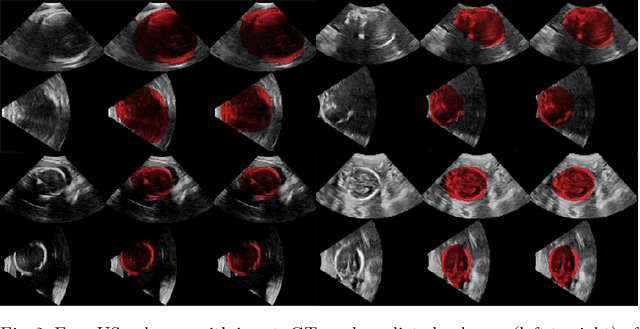
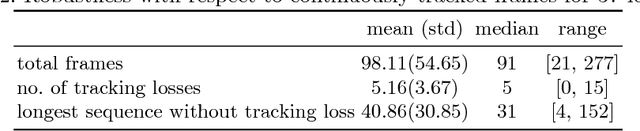
Abstract:Ultrasound (US) is the most widely used fetal imaging technique. However, US images have limited capture range, and suffer from view dependent artefacts such as acoustic shadows. Compounding of overlapping 3D US acquisitions into a high-resolution volume can extend the field of view and remove image artefacts, which is useful for retrospective analysis including population based studies. However, such volume reconstructions require information about relative transformations between probe positions from which the individual volumes were acquired. In prenatal US scans, the fetus can move independently from the mother, making external trackers such as electromagnetic or optical tracking unable to track the motion between probe position and the moving fetus. We provide a novel methodology for image-based tracking and volume reconstruction by combining recent advances in deep learning and simultaneous localisation and mapping (SLAM). Tracking semantics are established through the use of a Residual 3D U-Net and the output is fed to the SLAM algorithm. As a proof of concept, experiments are conducted on US volumes taken from a whole body fetal phantom, and from the heads of real fetuses. For the fetal head segmentation, we also introduce a novel weak annotation approach to minimise the required manual effort for ground truth annotation. We evaluate our method qualitatively, and quantitatively with respect to tissue discrimination accuracy and tracking robustness.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge