Cem M. Deniz
HIST-AID: Leveraging Historical Patient Reports for Enhanced Multi-Modal Automatic Diagnosis
Nov 16, 2024Abstract:Chest X-ray imaging is a widely accessible and non-invasive diagnostic tool for detecting thoracic abnormalities. While numerous AI models assist radiologists in interpreting these images, most overlook patients' historical data. To bridge this gap, we introduce Temporal MIMIC dataset, which integrates five years of patient history, including radiographic scans and reports from MIMIC-CXR and MIMIC-IV, encompassing 12,221 patients and thirteen pathologies. Building on this, we present HIST-AID, a framework that enhances automatic diagnostic accuracy using historical reports. HIST-AID emulates the radiologist's comprehensive approach, leveraging historical data to improve diagnostic accuracy. Our experiments demonstrate significant improvements, with AUROC increasing by 6.56% and AUPRC by 9.51% compared to models that rely solely on radiographic scans. These gains were consistently observed across diverse demographic groups, including variations in gender, age, and racial categories. We show that while recent data boost performance, older data may reduce accuracy due to changes in patient conditions. Our work paves the potential of incorporating historical data for more reliable automatic diagnosis, providing critical support for clinical decision-making.
Modified Risk Formulation for Improving the Prediction of Knee Osteoarthritis Progression
Jun 14, 2024Abstract:Current methods for predicting osteoarthritis (OA) outcomes do not incorporate disease specific prior knowledge to improve the outcome prediction models. We developed a novel approach that effectively uses consecutive imaging studies to improve OA outcome predictions by incorporating an OA severity constraint. This constraint ensures that the risk of OA for a knee should either increase or remain the same over time. DL models were trained to predict TKR within multiple time periods (1 year, 2 years, and 4 years) using knee radiographs and MRI scans. Models with and without the risk constraint were evaluated using the area under the receiver operator curve (AUROC) and the area under the precision recall curve (AUPRC) analysis. The novel RiskFORM2 method, leveraging a dual model risk constraint architecture, demonstrated superior performance, yielding an AUROC of 0.87 and AUPRC of 0.47 for 1 year TKR prediction on the OAI radiograph test set, a marked improvement over the 0.79 AUROC and 0.34 AUPRC of the baseline approach. The performance advantage extended to longer followup periods, with RiskFORM2 maintaining a high AUROC of 0.86 and AUPRC of 0.75 in predicting TKR within 4 years. Additionally, when generalizing to the external MOST radiograph test set, RiskFORM2 generalized better with an AUROC of 0.77 and AUPRC of 0.25 for 1 year predictions, which was higher than the 0.71 AUROC and 0.19 AUPRC of the baseline approach. In the MRI test sets, similar patterns emerged, with RiskFORM2 outperforming the baseline approach consistently. However, RiskFORM1 exhibited the highest AUROC of 0.86 and AUPRC of 0.72 for 4 year predictions on the OAI set.
MR-Transformer: Vision Transformer for Total Knee Replacement Prediction Using Magnetic Resonance Imaging
May 05, 2024Abstract:A transformer-based deep learning model, MR-Transformer, was developed for total knee replacement (TKR) prediction using magnetic resonance imaging (MRI). The model incorporates the ImageNet pre-training and captures three-dimensional (3D) spatial correlation from the MR images. The performance of the proposed model was compared to existing state-of-the-art deep learning models for knee injury diagnosis using MRI. Knee MR scans of four different tissue contrasts from the Osteoarthritis Initiative and Multicenter Osteoarthritis Study databases were utilized in the study. Experimental results demonstrated the state-of-the-art performance of the proposed model on TKR prediction using MRI.
Estimation of Time-to-Total Knee Replacement Surgery
Apr 29, 2024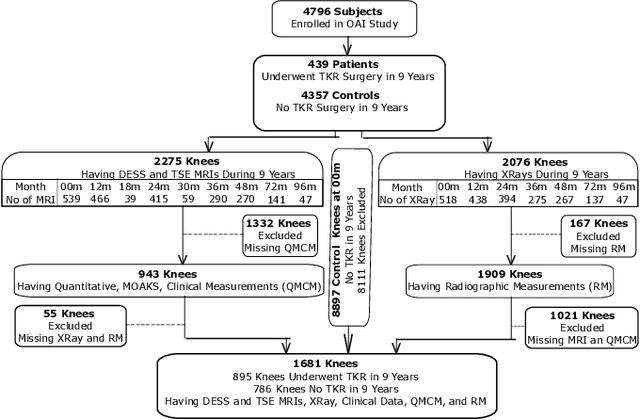
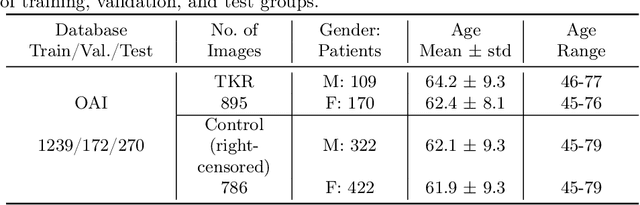
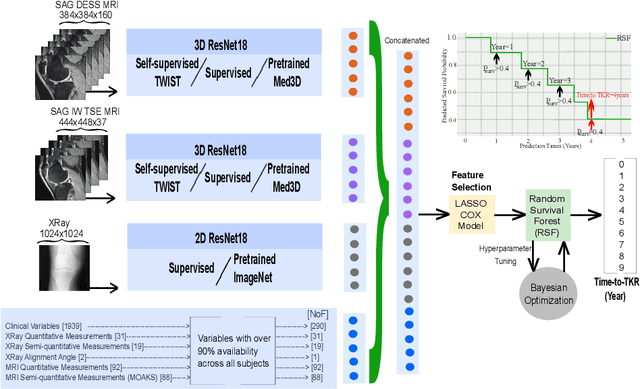
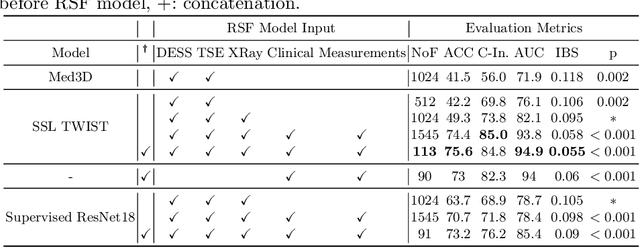
Abstract:A survival analysis model for predicting time-to-total knee replacement (TKR) was developed using features from medical images and clinical measurements. Supervised and self-supervised deep learning approaches were utilized to extract features from radiographs and magnetic resonance images. Extracted features were combined with clinical and image assessments for survival analysis using random survival forests. The proposed model demonstrated high discrimination power by combining deep learning features and clinical and image assessments using a fusion of multiple modalities. The model achieved an accuracy of 75.6% and a C-Index of 84.8% for predicting the time-to-TKR surgery. Accurate time-to-TKR predictions have the potential to help assist physicians to personalize treatment strategies and improve patient outcomes.
The International Workshop on Osteoarthritis Imaging Knee MRI Segmentation Challenge: A Multi-Institute Evaluation and Analysis Framework on a Standardized Dataset
May 26, 2020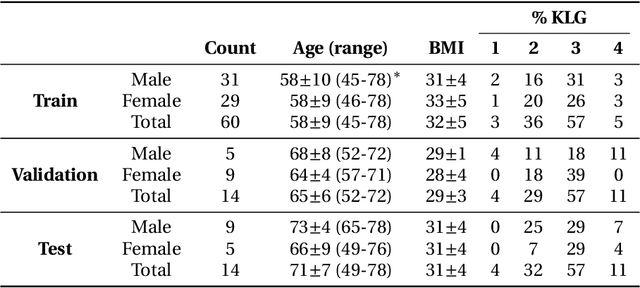
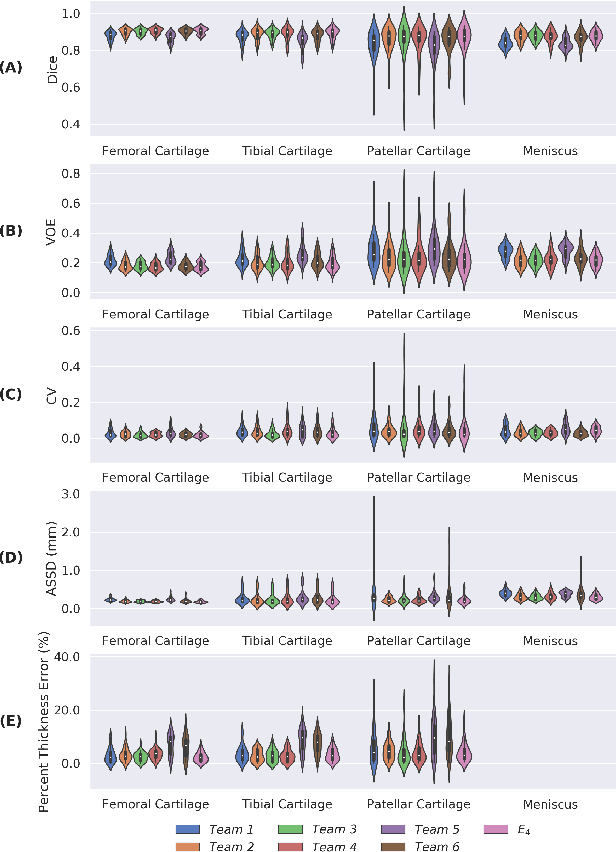
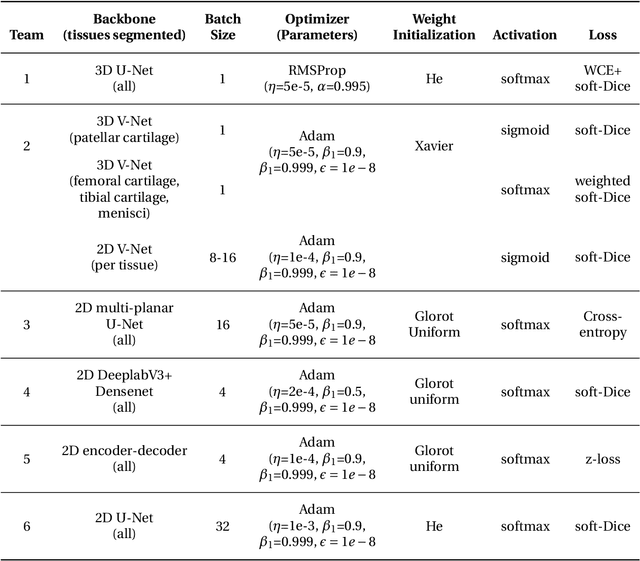
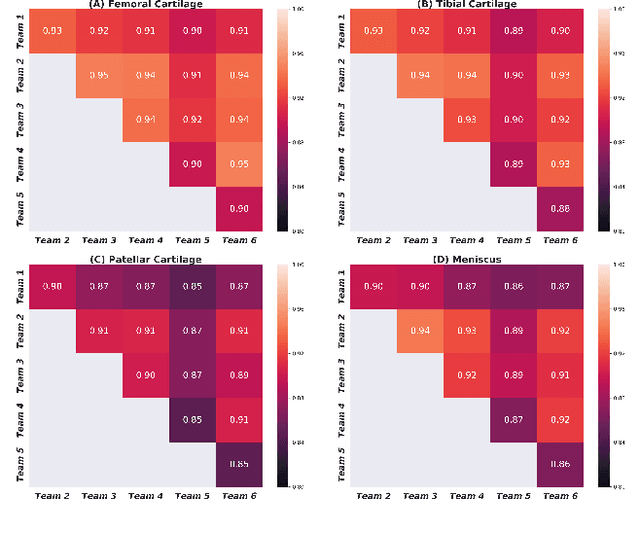
Abstract:Purpose: To organize a knee MRI segmentation challenge for characterizing the semantic and clinical efficacy of automatic segmentation methods relevant for monitoring osteoarthritis progression. Methods: A dataset partition consisting of 3D knee MRI from 88 subjects at two timepoints with ground-truth articular (femoral, tibial, patellar) cartilage and meniscus segmentations was standardized. Challenge submissions and a majority-vote ensemble were evaluated using Dice score, average symmetric surface distance, volumetric overlap error, and coefficient of variation on a hold-out test set. Similarities in network segmentations were evaluated using pairwise Dice correlations. Articular cartilage thickness was computed per-scan and longitudinally. Correlation between thickness error and segmentation metrics was measured using Pearson's coefficient. Two empirical upper bounds for ensemble performance were computed using combinations of model outputs that consolidated true positives and true negatives. Results: Six teams (T1-T6) submitted entries for the challenge. No significant differences were observed across all segmentation metrics for all tissues (p=1.0) among the four top-performing networks (T2, T3, T4, T6). Dice correlations between network pairs were high (>0.85). Per-scan thickness errors were negligible among T1-T4 (p=0.99) and longitudinal changes showed minimal bias (<0.03mm). Low correlations (<0.41) were observed between segmentation metrics and thickness error. The majority-vote ensemble was comparable to top performing networks (p=1.0). Empirical upper bound performances were similar for both combinations (p=1.0). Conclusion: Diverse networks learned to segment the knee similarly where high segmentation accuracy did not correlate to cartilage thickness accuracy. Voting ensembles did not outperform individual networks but may help regularize individual models.
Segmentation of the Proximal Femur from MR Images using Deep Convolutional Neural Networks
Mar 20, 2018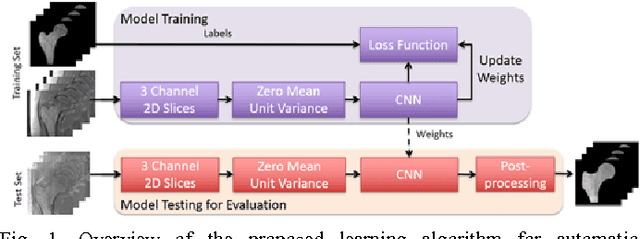
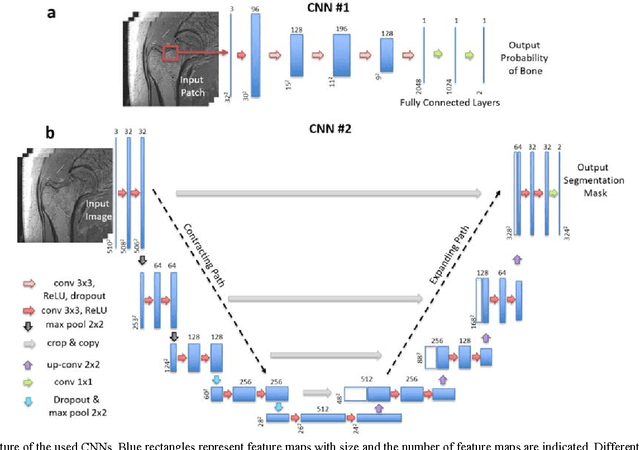
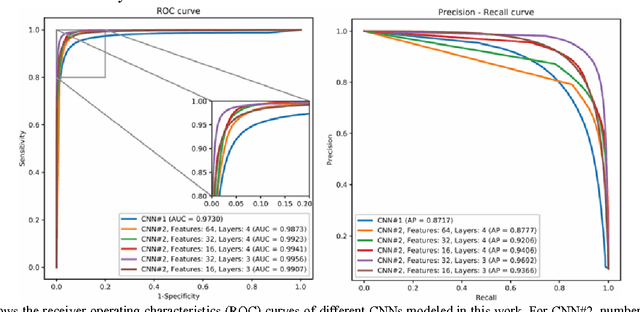
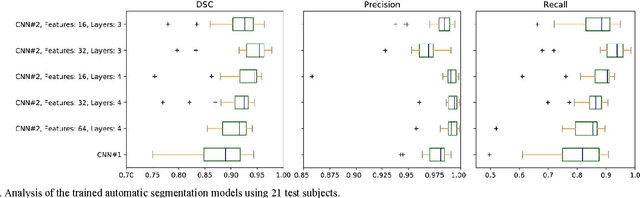
Abstract:Magnetic resonance imaging (MRI) has been proposed as a complimentary method to measure bone quality and assess fracture risk. However, manual segmentation of MR images of bone is time-consuming, limiting the use of MRI measurements in the clinical practice. The purpose of this paper is to present an automatic proximal femur segmentation method that is based on deep convolutional neural networks (CNNs). This study had institutional review board approval and written informed consent was obtained from all subjects. A dataset of volumetric structural MR images of the proximal femur from 86 subject were manually-segmented by an expert. We performed experiments by training two different CNN architectures with multiple number of initial feature maps and layers, and tested their segmentation performance against the gold standard of manual segmentations using four-fold cross-validation. Automatic segmentation of the proximal femur achieved a high dice similarity score of 0.94$\pm$0.05 with precision = 0.95$\pm$0.02, and recall = 0.94$\pm$0.08 using a CNN architecture based on 3D convolution exceeding the performance of 2D CNNs. The high segmentation accuracy provided by CNNs has the potential to help bring the use of structural MRI measurements of bone quality into clinical practice for management of osteoporosis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge