Richard Kijowski
Modified Risk Formulation for Improving the Prediction of Knee Osteoarthritis Progression
Jun 14, 2024Abstract:Current methods for predicting osteoarthritis (OA) outcomes do not incorporate disease specific prior knowledge to improve the outcome prediction models. We developed a novel approach that effectively uses consecutive imaging studies to improve OA outcome predictions by incorporating an OA severity constraint. This constraint ensures that the risk of OA for a knee should either increase or remain the same over time. DL models were trained to predict TKR within multiple time periods (1 year, 2 years, and 4 years) using knee radiographs and MRI scans. Models with and without the risk constraint were evaluated using the area under the receiver operator curve (AUROC) and the area under the precision recall curve (AUPRC) analysis. The novel RiskFORM2 method, leveraging a dual model risk constraint architecture, demonstrated superior performance, yielding an AUROC of 0.87 and AUPRC of 0.47 for 1 year TKR prediction on the OAI radiograph test set, a marked improvement over the 0.79 AUROC and 0.34 AUPRC of the baseline approach. The performance advantage extended to longer followup periods, with RiskFORM2 maintaining a high AUROC of 0.86 and AUPRC of 0.75 in predicting TKR within 4 years. Additionally, when generalizing to the external MOST radiograph test set, RiskFORM2 generalized better with an AUROC of 0.77 and AUPRC of 0.25 for 1 year predictions, which was higher than the 0.71 AUROC and 0.19 AUPRC of the baseline approach. In the MRI test sets, similar patterns emerged, with RiskFORM2 outperforming the baseline approach consistently. However, RiskFORM1 exhibited the highest AUROC of 0.86 and AUPRC of 0.72 for 4 year predictions on the OAI set.
MR-Transformer: Vision Transformer for Total Knee Replacement Prediction Using Magnetic Resonance Imaging
May 05, 2024Abstract:A transformer-based deep learning model, MR-Transformer, was developed for total knee replacement (TKR) prediction using magnetic resonance imaging (MRI). The model incorporates the ImageNet pre-training and captures three-dimensional (3D) spatial correlation from the MR images. The performance of the proposed model was compared to existing state-of-the-art deep learning models for knee injury diagnosis using MRI. Knee MR scans of four different tissue contrasts from the Osteoarthritis Initiative and Multicenter Osteoarthritis Study databases were utilized in the study. Experimental results demonstrated the state-of-the-art performance of the proposed model on TKR prediction using MRI.
Estimation of Time-to-Total Knee Replacement Surgery
Apr 29, 2024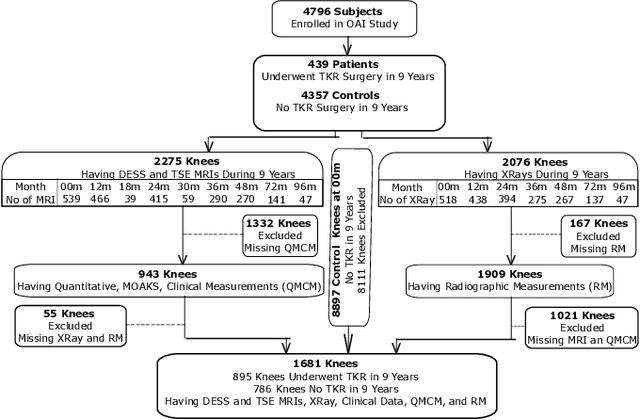
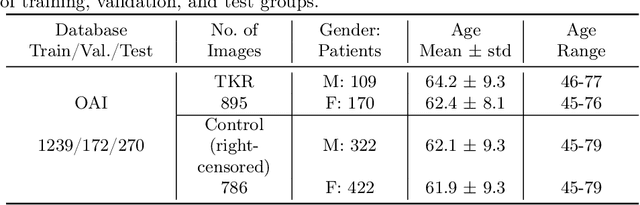
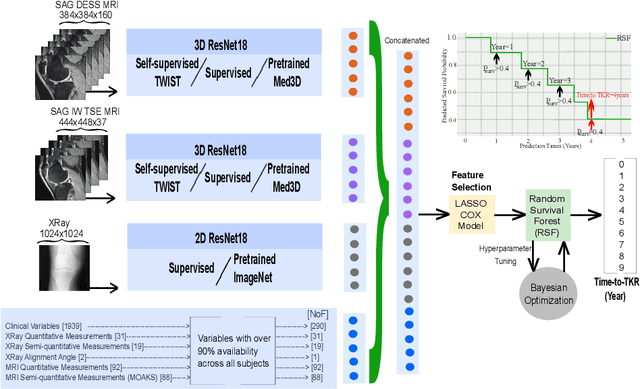
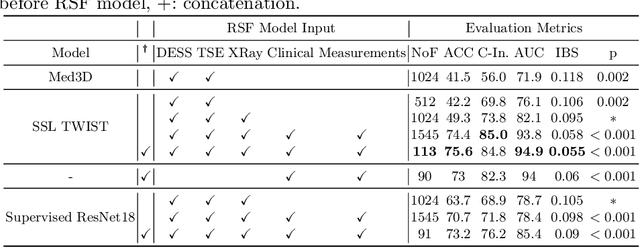
Abstract:A survival analysis model for predicting time-to-total knee replacement (TKR) was developed using features from medical images and clinical measurements. Supervised and self-supervised deep learning approaches were utilized to extract features from radiographs and magnetic resonance images. Extracted features were combined with clinical and image assessments for survival analysis using random survival forests. The proposed model demonstrated high discrimination power by combining deep learning features and clinical and image assessments using a fusion of multiple modalities. The model achieved an accuracy of 75.6% and a C-Index of 84.8% for predicting the time-to-TKR surgery. Accurate time-to-TKR predictions have the potential to help assist physicians to personalize treatment strategies and improve patient outcomes.
SpecNet: Spectral Domain Convolutional Neural Network
May 28, 2019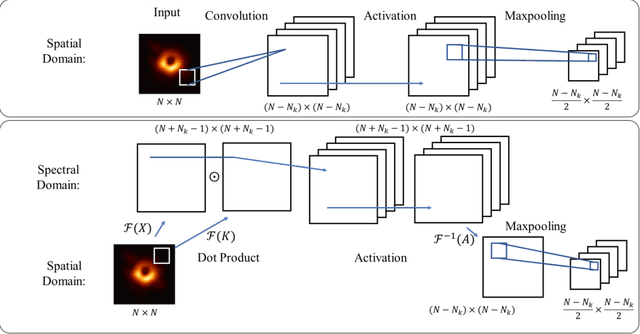
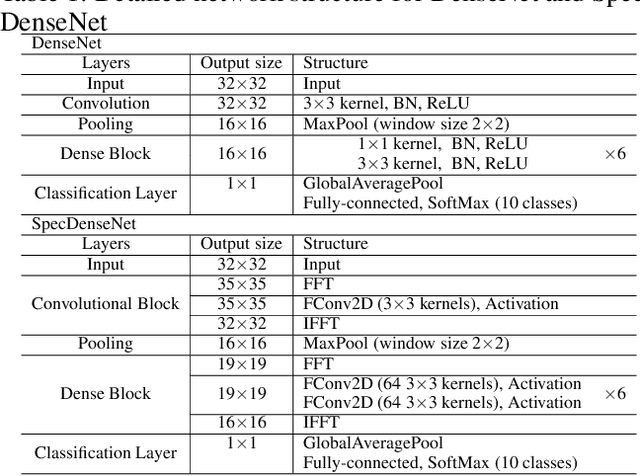

Abstract:The memory consumption of most Convolutional Neural Network (CNN) architectures grows rapidly with increasing depth of the network, which is a major constraint for efficient network training and inference on modern GPUs with yet limited memory. Several studies show that the feature maps (as generated after the convolutional layers) are the big bottleneck in this memory problem. Often, these feature maps mimic natural photographs in the sense that their energy is concentrated in the spectral domain. This paper proposes a Spectral Domain Convolutional Neural Network (SpecNet) that performs both the convolution and the activation operations in the spectral domain to achieve memory reduction. SpecNet exploits a configurable threshold to force small values in the feature maps to zero, allowing the feature maps to be stored sparsely. Since convolution in the spatial domain is equivalent to a dot product in the spectral domain, the multiplications only need to be performed on the non-zero entries of the (sparse) spectral domain feature maps. SpecNet also employs a special activation function that preserves the sparsity of the feature maps while effectively encouraging the convergence of the network. The performance of SpecNet is evaluated on three competitive object recognition benchmark tasks (MNIST, CIFAR-10, and SVHN), and compared with four state-of-the-art implementations (LeNet, AlexNet, VGG, and DenseNet). Overall, SpecNet is able to reduce memory consumption by about 60% without significant loss of performance for all tested network architectures.
SANTIS: Sampling-Augmented Neural neTwork with Incoherent Structure for MR image reconstruction
Dec 08, 2018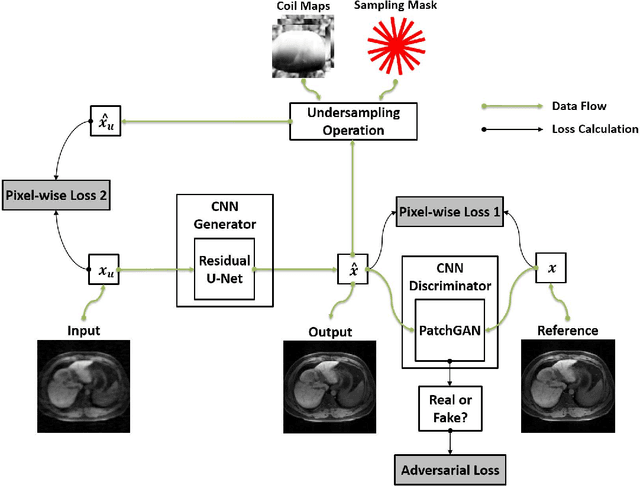
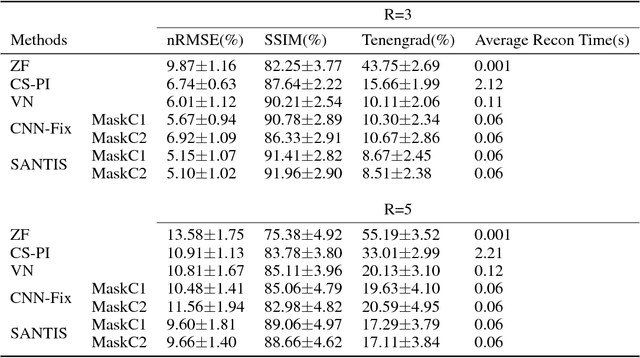
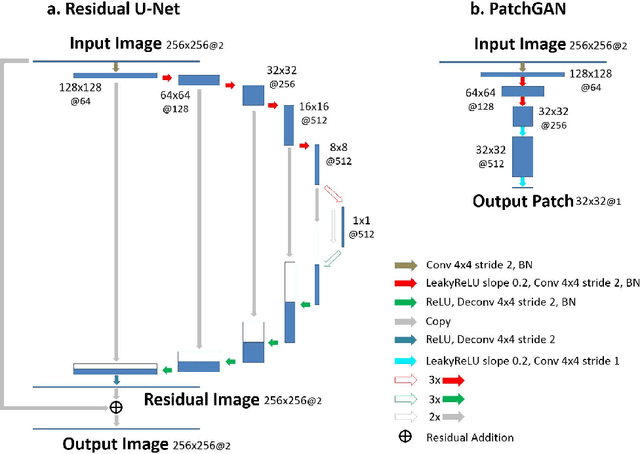
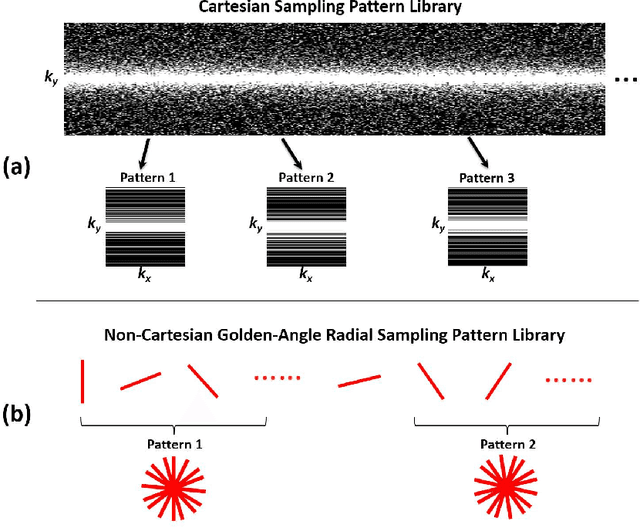
Abstract:Deep learning holds great promise in the reconstruction of undersampled Magnetic Resonance Imaging (MRI) data, providing new opportunities to escalate the performance of rapid MRI. In existing deep learning-based reconstruction methods, supervised training is performed using artifact-free reference images and their corresponding undersampled pairs. The undersampled images are generated by a fixed undersampling pattern in the training, and the trained network is then applied to reconstruct new images acquired with the same pattern in the inference. While such a training strategy can maintain a favorable reconstruction for a pre-selected undersampling pattern, the robustness of the trained network against any discrepancy of undersampling schemes is typically poor. We developed a novel deep learning-based reconstruction framework called SANTIS for efficient MR image reconstruction with improved robustness against sampling pattern discrepancy. SANTIS uses a data cycle-consistent adversarial network combining efficient end-to-end convolutional neural network mapping, data fidelity enforcement and adversarial training for reconstructing accelerated MR images more faithfully. A training strategy employing sampling augmentation with extensive variation of undersampling patterns was further introduced to promote the robustness of the trained network. Compared to conventional reconstruction and standard deep learning methods, SANTIS achieved consistent better reconstruction performance, with lower errors, greater image sharpness and higher similarity with respect to the reference regardless of the undersampling patterns during inference. This novel concept behind SANTIS can particularly be useful towards improving the robustness of deep learning-based image reconstruction against discrepancy between training and evaluation, which is currently an important but less studied open question.
MANTIS: Model-Augmented Neural neTwork with Incoherent k-space Sampling for efficient MR T2 mapping
Sep 02, 2018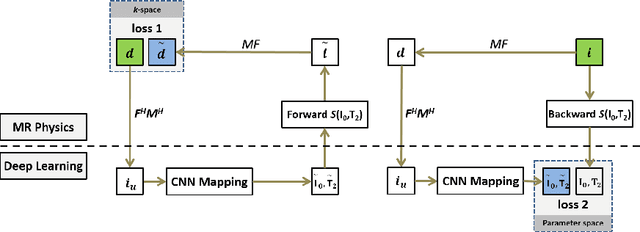

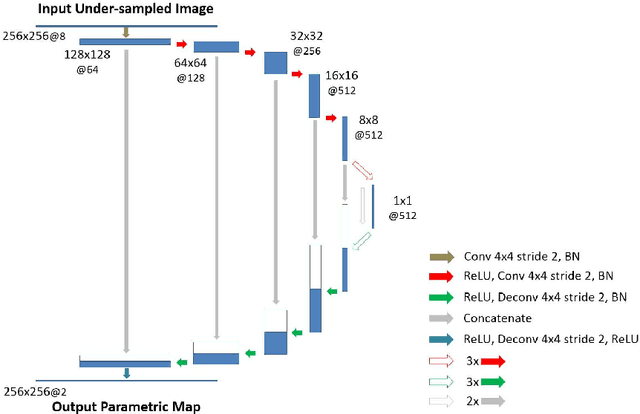

Abstract:Quantitative mapping of magnetic resonance (MR) parameters have been shown as valuable methods for improved assessment of a range of diseases. Due to the need to image an anatomic structure multiple times, parameter mapping usually requires long scan times compared to conventional static imaging. Therefore, accelerated parameter mapping is highly-desirable and remains a topic of great interest in the MR research community. While many recent deep learning methods have focused on highly efficient image reconstruction for conventional static MR imaging, applications of deep learning for dynamic imaging and in particular accelerated parameter mapping have been limited. The purpose of this work was to develop and evaluate a novel deep learning-based reconstruction framework called Model-Augmented Neural neTwork with Incoherent k-space Sampling (MANTIS) for efficient MR parameter mapping. Our approach combines end-to-end CNN mapping with k-space consistency using the concept of cyclic loss to further enforce data and model fidelity. Incoherent k-space sampling is used to improve reconstruction performance. A physical model is incorporated into the proposed framework, so that the parameter maps can be efficiently estimated directly from undersampled images. The performance of MANTIS was demonstrated for the spin-spin relaxation time (T2) mapping of the knee joint. Compared to conventional reconstruction approaches that exploited image sparsity, MANTIS yielded lower errors and higher similarity with respect to the reference in the T2 estimation. Our study demonstrated that the proposed MANTIS framework, with a combination of end-to-end CNN mapping, signal model-augmented data consistency, and incoherent k-space sampling, represents a promising approach for efficient MR parameter mapping. MANTIS can potentially be extended to other types of parameter mapping with appropriate models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge