Carole Sudre
Medical Imaging AI Competitions Lack Fairness
Dec 19, 2025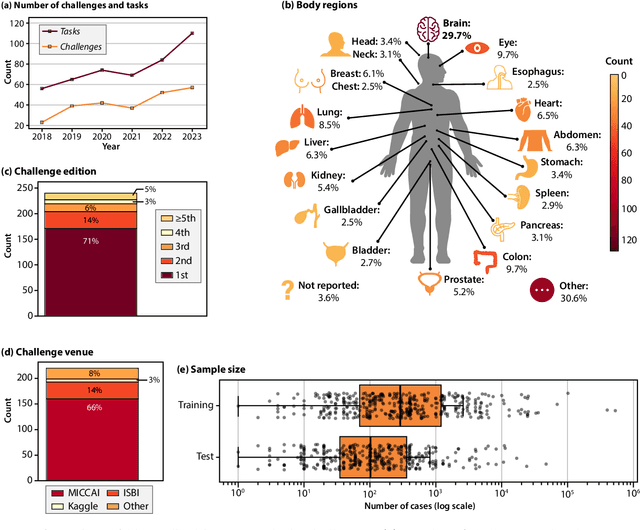
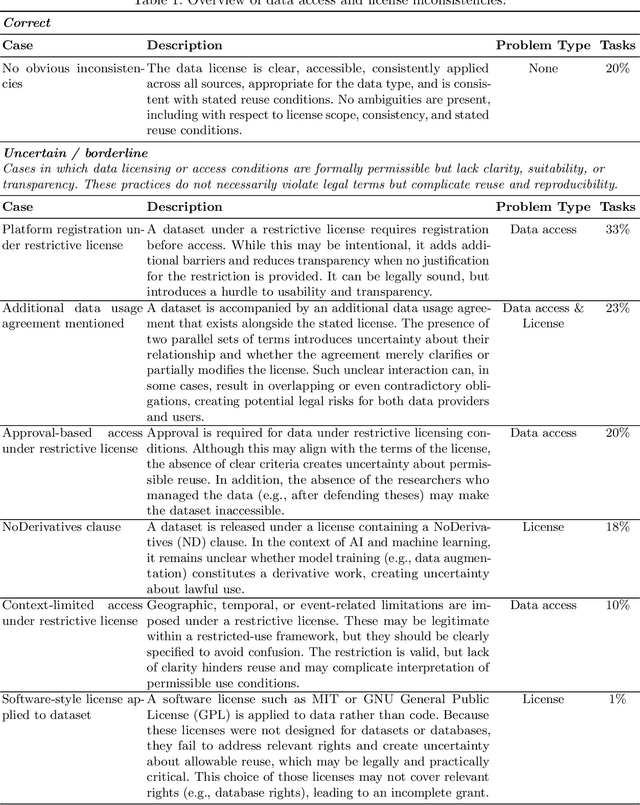
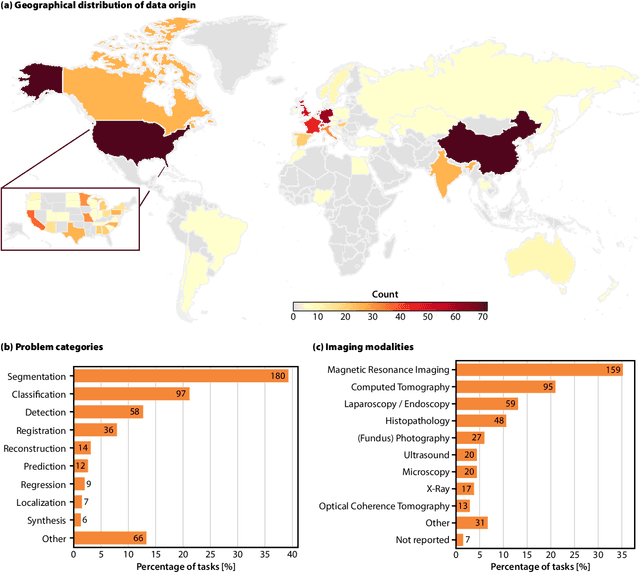
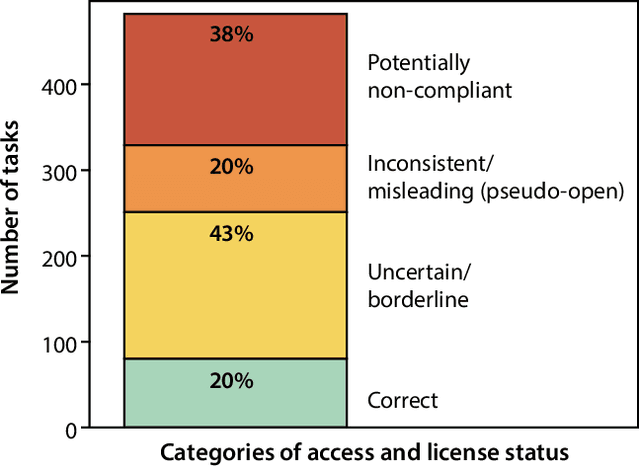
Abstract:Benchmarking competitions are central to the development of artificial intelligence (AI) in medical imaging, defining performance standards and shaping methodological progress. However, it remains unclear whether these benchmarks provide data that are sufficiently representative, accessible, and reusable to support clinically meaningful AI. In this work, we assess fairness along two complementary dimensions: (1) whether challenge datasets are representative of real-world clinical diversity, and (2) whether they are accessible and legally reusable in line with the FAIR principles. To address this question, we conducted a large-scale systematic study of 241 biomedical image analysis challenges comprising 458 tasks across 19 imaging modalities. Our findings show substantial biases in dataset composition, including geographic location, modality-, and problem type-related biases, indicating that current benchmarks do not adequately reflect real-world clinical diversity. Despite their widespread influence, challenge datasets were frequently constrained by restrictive or ambiguous access conditions, inconsistent or non-compliant licensing practices, and incomplete documentation, limiting reproducibility and long-term reuse. Together, these shortcomings expose foundational fairness limitations in our benchmarking ecosystem and highlight a disconnect between leaderboard success and clinical relevance.
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Segmenting white matter hyperintensities on isotropic three-dimensional Fluid Attenuated Inversion Recovery magnetic resonance images: A comparison of Deep learning tools on a Norwegian national imaging database
Jul 19, 2022



Abstract:Automated segmentation of white matter hyperintensities (WMHs) is an essential step in neuroimaging analysis of Magnetic Resonance Imaging (MRI). Fluid Attenuated Inversion Recovery (FLAIR-weighted) is an MRI contrast that is particularly useful to visualize and quantify WMHs, a hallmark of cerebral small vessel disease and Alzheimer's disease (AD). Clinical MRI protocols migrate to a three-dimensional (3D) FLAIR-weighted acquisition to enable high spatial resolution in all three voxel dimensions. The current study details the deployment of deep learning tools to enable automated WMH segmentation and characterization from 3D FLAIR-weighted images acquired as part of a national AD imaging initiative. Among 642 participants (283 male, mean age: (65.18 +/- 9.33) years) from the DDI study, two in-house networks were trained and validated across five national collection sites. Three models were tested on a held-out subset of the internal data from the 642 participants and an external dataset with 29 cases from an international collaborator. These test sets were evaluated independently. Five established WMH performance metrics were used for comparison against ground truth human-in-the-loop segmentation. Results of the three networks tested, the 3D nnU-Net had the best performance with an average dice similarity coefficient score of 0.78 +/- 0.10, performing better than both the in-house developed 2.5D model and the SOTA Deep Bayesian network. With the increasing use of 3D FLAIR-weighted images in MRI protocols, our results suggest that WMH segmentation models can be trained on 3D data and yield WMH segmentation performance that is comparable to or better than state-of-the-art without the need for including T1-weighted image series.
Evaluation of automated airway morphological quantification for assessing fibrosing lung disease
Nov 19, 2021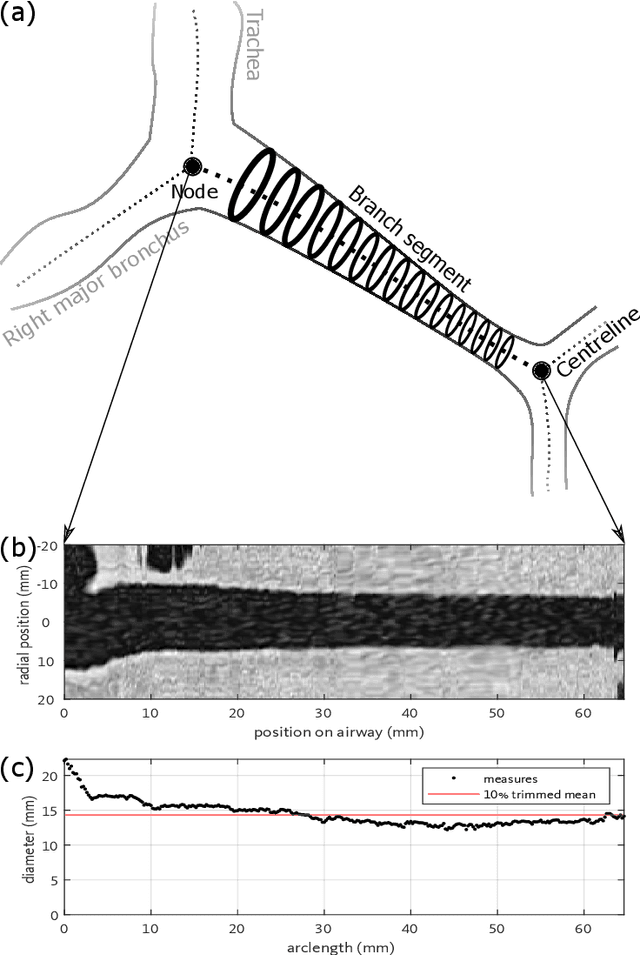
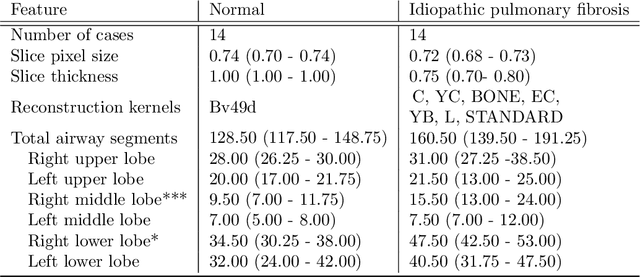
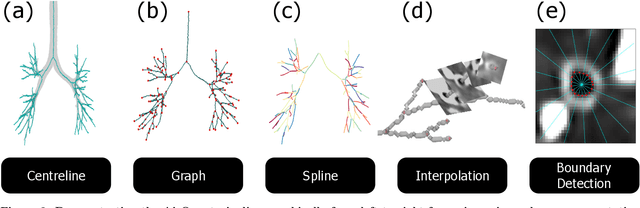
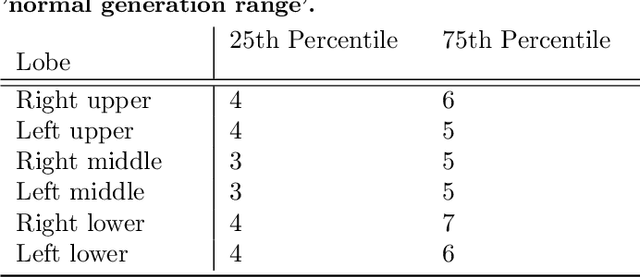
Abstract:Abnormal airway dilatation, termed traction bronchiectasis, is a typical feature of idiopathic pulmonary fibrosis (IPF). Volumetric computed tomography (CT) imaging captures the loss of normal airway tapering in IPF. We postulated that automated quantification of airway abnormalities could provide estimates of IPF disease extent and severity. We propose AirQuant, an automated computational pipeline that systematically parcellates the airway tree into its lobes and generational branches from a deep learning based airway segmentation, deriving airway structural measures from chest CT. Importantly, AirQuant prevents the occurrence of spurious airway branches by thick wave propagation and removes loops in the airway-tree by graph search, overcoming limitations of existing airway skeletonisation algorithms. Tapering between airway segments (intertapering) and airway tortuosity computed by AirQuant were compared between 14 healthy participants and 14 IPF patients. Airway intertapering was significantly reduced in IPF patients, and airway tortuosity was significantly increased when compared to healthy controls. Differences were most marked in the lower lobes, conforming to the typical distribution of IPF-related damage. AirQuant is an open-source pipeline that avoids limitations of existing airway quantification algorithms and has clinical interpretability. Automated airway measurements may have potential as novel imaging biomarkers of IPF severity and disease extent.
ICAM-reg: Interpretable Classification and Regression with Feature Attribution for Mapping Neurological Phenotypes in Individual Scans
Mar 03, 2021



Abstract:An important goal of medical imaging is to be able to precisely detect patterns of disease specific to individual scans; however, this is challenged in brain imaging by the degree of heterogeneity of shape and appearance. Traditional methods, based on image registration to a global template, historically fail to detect variable features of disease, as they utilise population-based analyses, suited primarily to studying group-average effects. In this paper we therefore take advantage of recent developments in generative deep learning to develop a method for simultaneous classification, or regression, and feature attribution (FA). Specifically, we explore the use of a VAE-GAN translation network called ICAM, to explicitly disentangle class relevant features from background confounds for improved interpretability and regression of neurological phenotypes. We validate our method on the tasks of Mini-Mental State Examination (MMSE) cognitive test score prediction for the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort, as well as brain age prediction, for both neurodevelopment and neurodegeneration, using the developing Human Connectome Project (dHCP) and UK Biobank datasets. We show that the generated FA maps can be used to explain outlier predictions and demonstrate that the inclusion of a regression module improves the disentanglement of the latent space. Our code is freely available on Github https://github.com/CherBass/ICAM.
ICAM: Interpretable Classification via Disentangled Representations and Feature Attribution Mapping
Jun 16, 2020



Abstract:Feature attribution (FA), or the assignment of class-relevance to different locations in an image, is important for many classification problems but is particularly crucial within the neuroscience domain, where accurate mechanistic models of behaviours, or disease, require knowledge of all features discriminative of a trait. At the same time, predicting class relevance from brain images is challenging as phenotypes are typically heterogeneous, and changes occur against a background of significant natural variation. Here, we present a novel framework for creating class specific FA maps through image-to-image translation. We propose the use of a VAE-GAN to explicitly disentangle class relevance from background features for improved interpretability properties, which results in meaningful FA maps. We validate our method on 2D and 3D brain image datasets of dementia (ADNI dataset), ageing (UK Biobank), and (simulated) lesion detection. We show that FA maps generated by our method outperform baseline FA methods when validated against ground truth. More significantly, our approach is the first to use latent space sampling to support exploration of phenotype variation. Our code will be available online at https://github.com/CherBass/ICAM.
NiftyNet: a deep-learning platform for medical imaging
Oct 16, 2017



Abstract:Medical image analysis and computer-assisted intervention problems are increasingly being addressed with deep-learning-based solutions. Established deep-learning platforms are flexible but do not provide specific functionality for medical image analysis and adapting them for this application requires substantial implementation effort. Thus, there has been substantial duplication of effort and incompatible infrastructure developed across many research groups. This work presents the open-source NiftyNet platform for deep learning in medical imaging. The ambition of NiftyNet is to accelerate and simplify the development of these solutions, and to provide a common mechanism for disseminating research outputs for the community to use, adapt and build upon. NiftyNet provides a modular deep-learning pipeline for a range of medical imaging applications including segmentation, regression, image generation and representation learning applications. Components of the NiftyNet pipeline including data loading, data augmentation, network architectures, loss functions and evaluation metrics are tailored to, and take advantage of, the idiosyncracies of medical image analysis and computer-assisted intervention. NiftyNet is built on TensorFlow and supports TensorBoard visualization of 2D and 3D images and computational graphs by default. We present 3 illustrative medical image analysis applications built using NiftyNet: (1) segmentation of multiple abdominal organs from computed tomography; (2) image regression to predict computed tomography attenuation maps from brain magnetic resonance images; and (3) generation of simulated ultrasound images for specified anatomical poses. NiftyNet enables researchers to rapidly develop and distribute deep learning solutions for segmentation, regression, image generation and representation learning applications, or extend the platform to new applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge