Wing Keung Cheung
A data-centric deep learning approach to airway segmentation
Jul 29, 2023Abstract:The morphology and distribution of airway tree abnormalities enables diagnosis and disease characterisation across a variety of chronic respiratory conditions. In this regard, airway segmentation plays a critical role in the production of the outline of the entire airway tree to enable estimation of disease extent and severity. In this study, we propose a data-centric deep learning technique to segment the airway tree. The proposed technique utilises interpolation and image split to improve data usefulness and quality. Then, an ensemble learning strategy is implemented to aggregate the segmented airway trees at different scales. In terms of segmentation performance (dice similarity coefficient), our method outperforms the baseline model by 2.5% on average when a combined loss is used. Further, our proposed technique has a low GPU usage and high flexibility enabling it to be deployed on any 2D deep learning model.
A 3D deep learning classifier and its explainability when assessing coronary artery disease
Jul 29, 2023Abstract:Early detection and diagnosis of coronary artery disease (CAD) could save lives and reduce healthcare costs. In this study, we propose a 3D Resnet-50 deep learning model to directly classify normal subjects and CAD patients on computed tomography coronary angiography images. Our proposed method outperforms a 2D Resnet-50 model by 23.65%. Explainability is also provided by using a Grad-GAM. Furthermore, we link the 3D CAD classification to a 2D two-class semantic segmentation for improved explainability and accurate abnormality localisation.
Airway measurement by refinement of synthetic images improves mortality prediction in idiopathic pulmonary fibrosis
Aug 30, 2022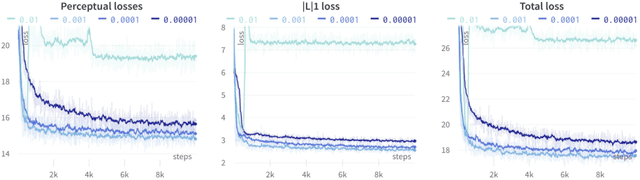
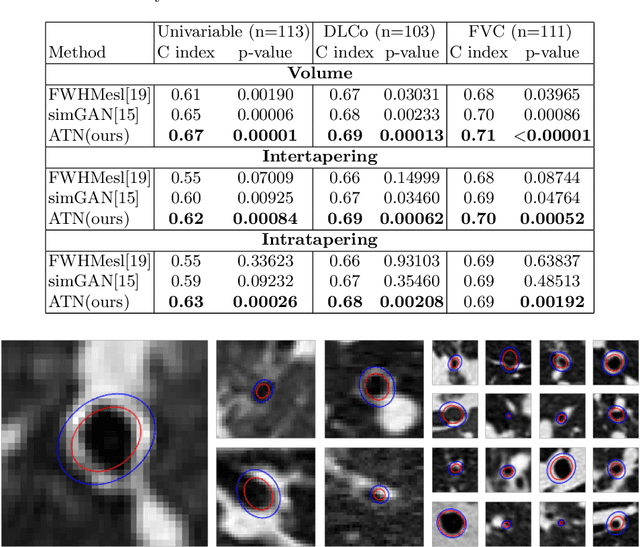
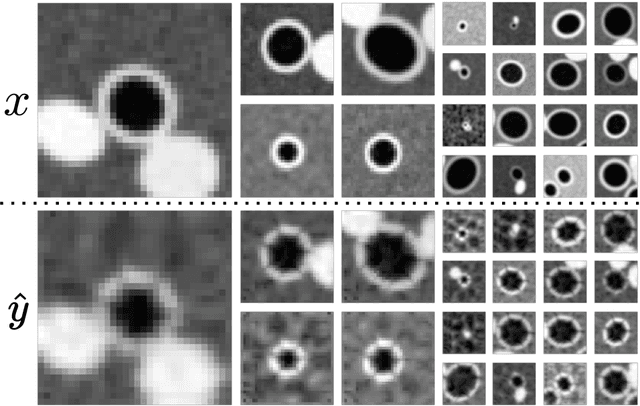
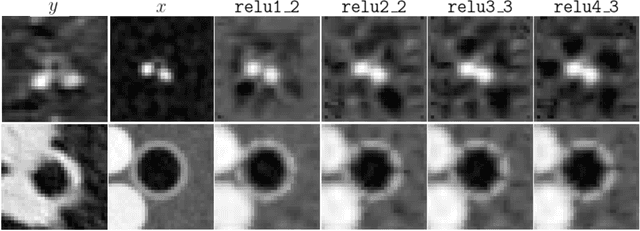
Abstract:Several chronic lung diseases, like idiopathic pulmonary fibrosis (IPF) are characterised by abnormal dilatation of the airways. Quantification of airway features on computed tomography (CT) can help characterise disease progression. Physics based airway measurement algorithms have been developed, but have met with limited success in part due to the sheer diversity of airway morphology seen in clinical practice. Supervised learning methods are also not feasible due to the high cost of obtaining precise airway annotations. We propose synthesising airways by style transfer using perceptual losses to train our model, Airway Transfer Network (ATN). We compare our ATN model with a state-of-the-art GAN-based network (simGAN) using a) qualitative assessment; b) assessment of the ability of ATN and simGAN based CT airway metrics to predict mortality in a population of 113 patients with IPF. ATN was shown to be quicker and easier to train than simGAN. ATN-based airway measurements were also found to be consistently stronger predictors of mortality than simGAN-derived airway metrics on IPF CTs. Airway synthesis by a transformation network that refines synthetic data using perceptual losses is a realistic alternative to GAN-based methods for clinical CT analyses of idiopathic pulmonary fibrosis. Our source code can be found at https://github.com/ashkanpakzad/ATN that is compatible with the existing open-source airway analysis framework, AirQuant.
Evaluation of automated airway morphological quantification for assessing fibrosing lung disease
Nov 19, 2021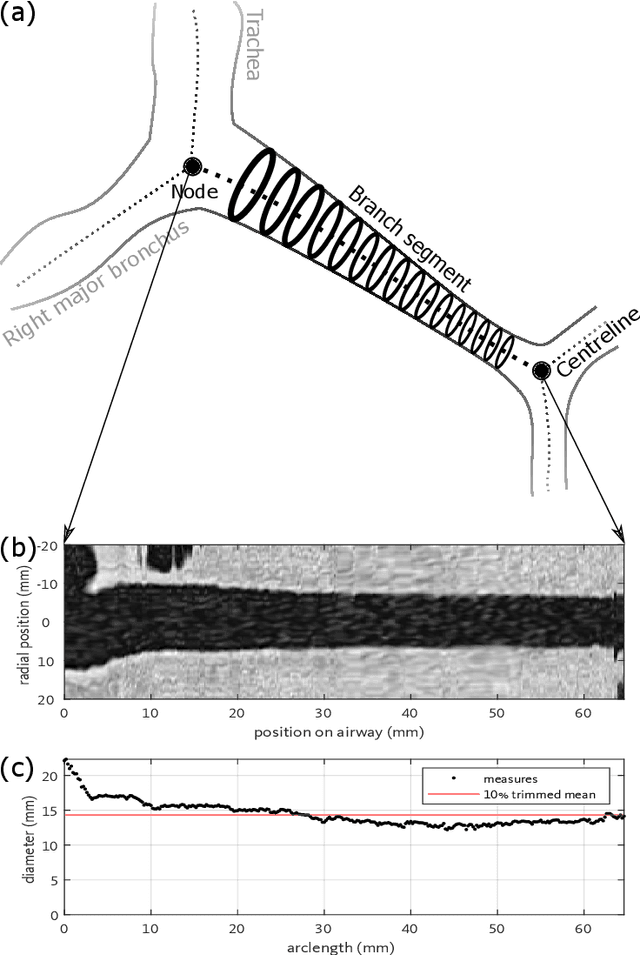
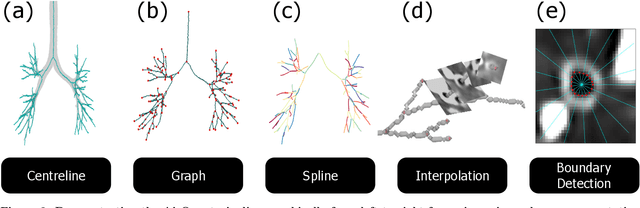
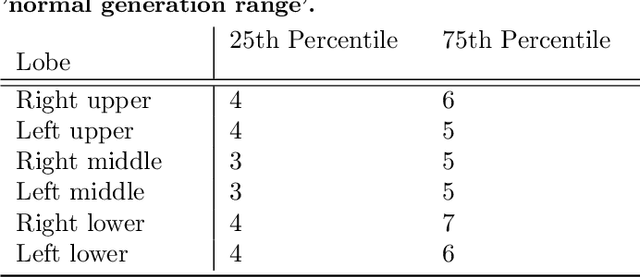
Abstract:Abnormal airway dilatation, termed traction bronchiectasis, is a typical feature of idiopathic pulmonary fibrosis (IPF). Volumetric computed tomography (CT) imaging captures the loss of normal airway tapering in IPF. We postulated that automated quantification of airway abnormalities could provide estimates of IPF disease extent and severity. We propose AirQuant, an automated computational pipeline that systematically parcellates the airway tree into its lobes and generational branches from a deep learning based airway segmentation, deriving airway structural measures from chest CT. Importantly, AirQuant prevents the occurrence of spurious airway branches by thick wave propagation and removes loops in the airway-tree by graph search, overcoming limitations of existing airway skeletonisation algorithms. Tapering between airway segments (intertapering) and airway tortuosity computed by AirQuant were compared between 14 healthy participants and 14 IPF patients. Airway intertapering was significantly reduced in IPF patients, and airway tortuosity was significantly increased when compared to healthy controls. Differences were most marked in the lower lobes, conforming to the typical distribution of IPF-related damage. AirQuant is an open-source pipeline that avoids limitations of existing airway quantification algorithms and has clinical interpretability. Automated airway measurements may have potential as novel imaging biomarkers of IPF severity and disease extent.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge