Kin Quan
Evaluation of automated airway morphological quantification for assessing fibrosing lung disease
Nov 19, 2021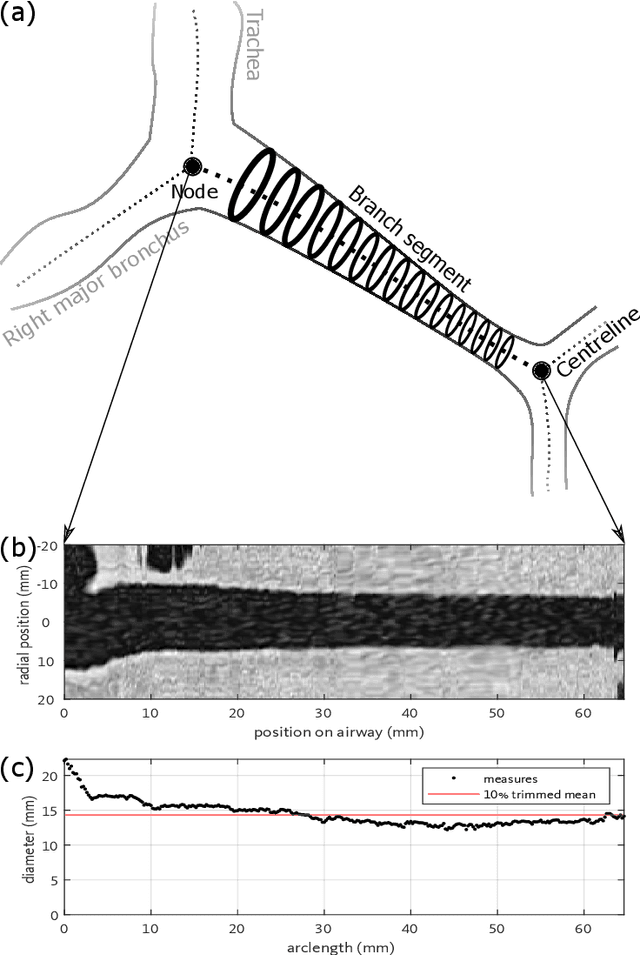
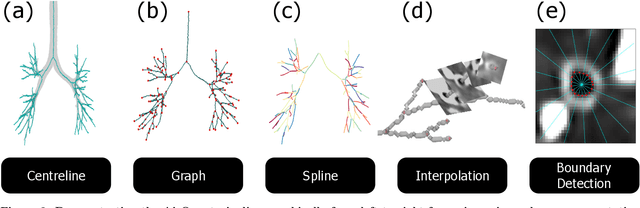
Abstract:Abnormal airway dilatation, termed traction bronchiectasis, is a typical feature of idiopathic pulmonary fibrosis (IPF). Volumetric computed tomography (CT) imaging captures the loss of normal airway tapering in IPF. We postulated that automated quantification of airway abnormalities could provide estimates of IPF disease extent and severity. We propose AirQuant, an automated computational pipeline that systematically parcellates the airway tree into its lobes and generational branches from a deep learning based airway segmentation, deriving airway structural measures from chest CT. Importantly, AirQuant prevents the occurrence of spurious airway branches by thick wave propagation and removes loops in the airway-tree by graph search, overcoming limitations of existing airway skeletonisation algorithms. Tapering between airway segments (intertapering) and airway tortuosity computed by AirQuant were compared between 14 healthy participants and 14 IPF patients. Airway intertapering was significantly reduced in IPF patients, and airway tortuosity was significantly increased when compared to healthy controls. Differences were most marked in the lower lobes, conforming to the typical distribution of IPF-related damage. AirQuant is an open-source pipeline that avoids limitations of existing airway quantification algorithms and has clinical interpretability. Automated airway measurements may have potential as novel imaging biomarkers of IPF severity and disease extent.
Reproducibility of an airway tapering measurement in CT with application to bronchiectasis
Sep 16, 2019



Abstract:Purpose: This paper proposes a pipeline to acquire a scalar tapering measurement from the carina to the most distal point of an individual airway visible on CT. We show the applicability of using tapering measurements on clinically acquired data by quantifying the reproducibility of the tapering measure. Methods: We generate a spline from the centreline of an airway to measure the area and arclength at contiguous intervals. The tapering measurement is the gradient of the linear regression between area in log space and arclength. The reproducibility of the measure was assessed by analysing different radiation doses, voxel sizes and reconstruction kernel on single timepoint and longitudinal CT scans and by evaluating the effct of airway bifurcations. Results: Using 74 airways from 10 CT scans, we show a statistical difference, p = 3.4 $\times$ 10$^{-4}$ in tapering between healthy airways (n = 35) and those affected by bronchiectasis (n = 39). The difference between the mean of the two populations was 0.011mm$^{-1}$ and the difference between the medians of the two populations was 0.006mm$^{-1}$. The tapering measurement retained a 95\% confidence interval of $\pm$0.005mm$^{-1}$ in a simulated 25 mAs scan and retained a 95% confidence of $\pm$0.005mm$^{-1}$ on simulated CTs up to 1.5 times the original voxel size. Conclusion: We have established an estimate of the precision of the tapering measurement and estimated the effect on precision of simulated voxel size and CT scan dose. We recommend that the scanner calibration be undertaken with the phantoms as described, on the specific CT scanner, radiation dose and reconstruction algorithm that is to be used in any quantitative studies. Our code is available at https://github.com/quan14/AirwayTaperingInCT
* 55 pages, 18 figures, The manuscript was originally published in Journal of Medical Imaging
Tapering Analysis of Airways with Bronchiectasis
Sep 14, 2019Abstract:Bronchiectasis is the permanent dilation of airways. Patients with the disease can suffer recurrent exacerbations, reducing their quality of life. The gold standard to diagnose and monitor bronchiectasis is accomplished by inspection of chest computed tomography (CT) scans. A clinician examines the broncho-arterial ratio to determine if an airway is brochiectatic. The visual analysis assumes the blood vessel diameter remains constant, although this assumption is disputed in the literature. We propose a simple measurement of tapering along the airways to diagnose and monitor bronchiectasis. To this end, we constructed a pipeline to measure the cross-sectional area along the airways at contiguous intervals, starting from the carina to the most distal point observable. Using a phantom with calibrated 3D printed structures, the precision and accuracy of our algorithm extends to the sub voxel level. The tapering measurement is robust to bifurcations along the airway and was applied to chest CT images acquired in clinical practice. The result is a statistical difference in tapering rate between airways with bronchiectasis and controls. Our code is available at https://github.com/quan14/AirwayTaperingInCT.
* 12 pages, 7 figures. Previously submitted for SPIE Medical Imaging, 2018, Houston, Texas, United States
Modelling Airway Geometry as Stock Market Data using Bayesian Changepoint Detection
Jun 28, 2019



Abstract:Numerous lung diseases, such as idiopathic pulmonary fibrosis (IPF), exhibit dilation of the airways. Accurate measurement of dilatation enables assessment of the progression of disease. Unfortunately the combination of image noise and airway bifurcations causes high variability in the profiles of cross-sectional areas, rendering the identification of affected regions very difficult. Here we introduce a noise-robust method for automatically detecting the location of progressive airway dilatation given two profiles of the same airway acquired at different time points. We propose a probabilistic model of abrupt relative variations between profiles and perform inference via Reversible Jump Markov Chain Monte Carlo sampling. We demonstrate the efficacy of the proposed method on two datasets; (i) images of healthy airways with simulated dilatation; (ii) pairs of real images of IPF-affected airways acquired at 1 year intervals. Our model is able to detect the starting location of airway dilatation with an accuracy of 2.5mm on simulated data. The experiments on the IPF dataset display reasonable agreement with radiologists. We can compute a relative change in airway volume that may be useful for quantifying IPF disease progression.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge