Andrew Beers
Semi-Supervised Deep Learning for Abnormality Classification in Retinal Images
Dec 19, 2018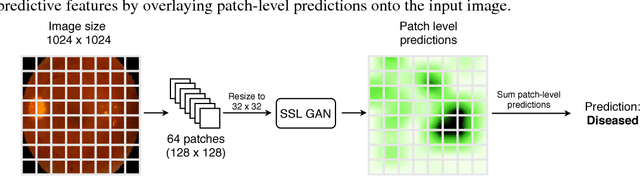


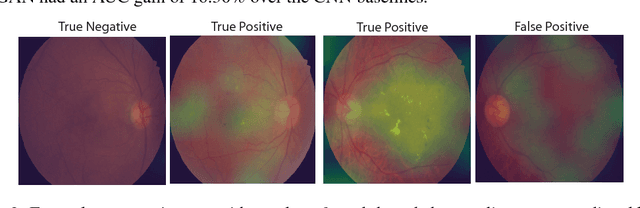
Abstract:Supervised deep learning algorithms have enabled significant performance gains in medical image classification tasks. But these methods rely on large labeled datasets that require resource-intensive expert annotation. Semi-supervised generative adversarial network (GAN) approaches offer a means to learn from limited labeled data alongside larger unlabeled datasets, but have not been applied to discern fine-scale, sparse or localized features that define medical abnormalities. To overcome these limitations, we propose a patch-based semi-supervised learning approach and evaluate performance on classification of diabetic retinopathy from funduscopic images. Our semi-supervised approach achieves high AUC with just 10-20 labeled training images, and outperforms the supervised baselines by upto 15% when less than 30% of the training dataset is labeled. Further, our method implicitly enables interpretation of the SSL predictions. As this approach enables good accuracy, resolution and interpretability with lower annotation burden, it sets the pathway for scalable applications of deep learning in clinical imaging.
Deep feature transfer between localization and segmentation tasks
Nov 10, 2018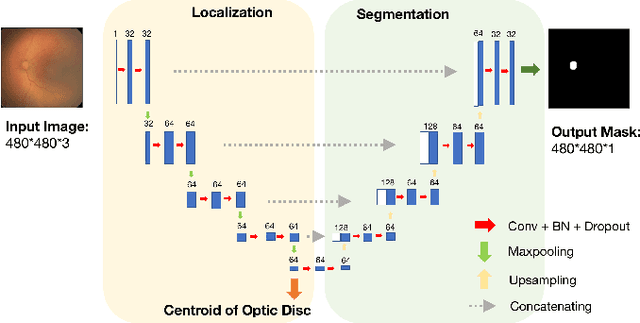
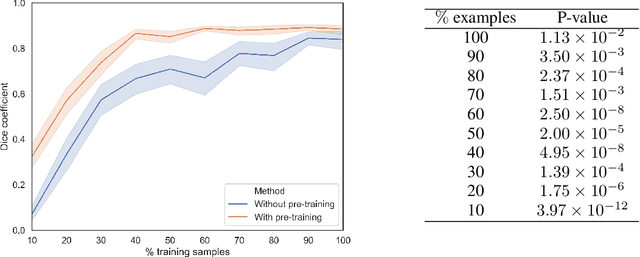
Abstract:In this paper, we propose a new pre-training scheme for U-net based image segmentation. We first train the encoding arm as a localization network to predict the center of the target, before extending it into a U-net architecture for segmentation. We apply our proposed method to the problem of segmenting the optic disc from fundus photographs. Our work shows that the features learned by encoding arm can be transferred to the segmentation network to reduce the annotation burden. We propose that an approach could have broad utility for medical image segmentation, and alleviate the burden of delineating complex structures by pre-training on annotations that are much easier to acquire.
DeepNeuro: an open-source deep learning toolbox for neuroimaging
Aug 14, 2018
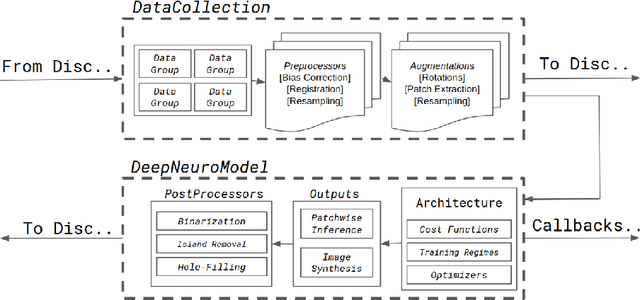
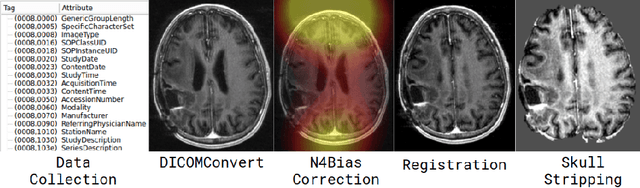
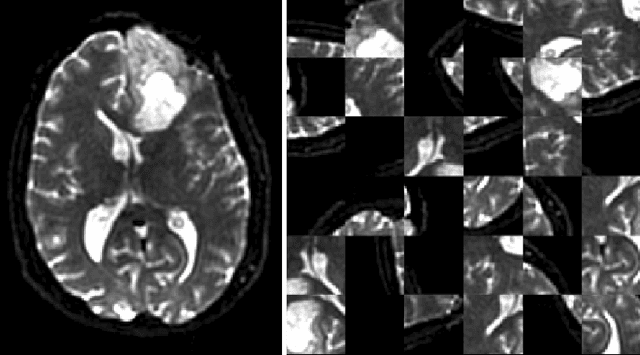
Abstract:Translating neural networks from theory to clinical practice has unique challenges, specifically in the field of neuroimaging. In this paper, we present DeepNeuro, a deep learning framework that is best-suited to putting deep learning algorithms for neuroimaging in practical usage with a minimum of friction. We show how this framework can be used to both design and train neural network architectures, as well as modify state-of-the-art architectures in a flexible and intuitive way. We display the pre- and postprocessing functions common in the medical imaging community that DeepNeuro offers to ensure consistent performance of networks across variable users, institutions, and scanners. And we show how pipelines created in DeepNeuro can be concisely packaged into shareable Docker containers and command-line interfaces using DeepNeuro's pipeline resources.
High-resolution medical image synthesis using progressively grown generative adversarial networks
May 09, 2018
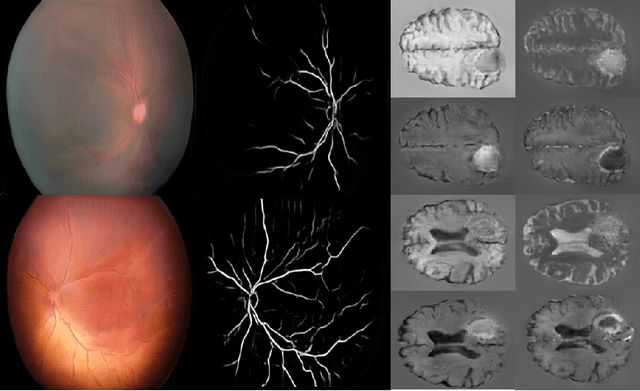
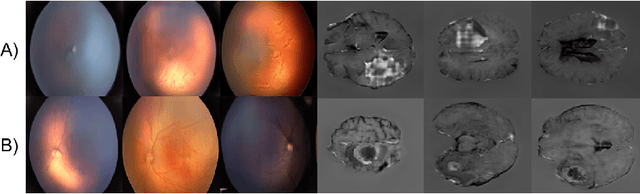
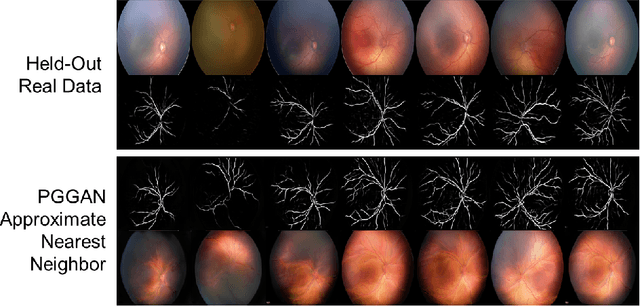
Abstract:Generative adversarial networks (GANs) are a class of unsupervised machine learning algorithms that can produce realistic images from randomly-sampled vectors in a multi-dimensional space. Until recently, it was not possible to generate realistic high-resolution images using GANs, which has limited their applicability to medical images that contain biomarkers only detectable at native resolution. Progressive growing of GANs is an approach wherein an image generator is trained to initially synthesize low resolution synthetic images (8x8 pixels), which are then fed to a discriminator that distinguishes these synthetic images from real downsampled images. Additional convolutional layers are then iteratively introduced to produce images at twice the previous resolution until the desired resolution is reached. In this work, we demonstrate that this approach can produce realistic medical images in two different domains; fundus photographs exhibiting vascular pathology associated with retinopathy of prematurity (ROP), and multi-modal magnetic resonance images of glioma. We also show that fine-grained details associated with pathology, such as retinal vessels or tumor heterogeneity, can be preserved and enhanced by including segmentation maps as additional channels. We envisage several applications of the approach, including image augmentation and unsupervised classification of pathology.
Institutionally Distributed Deep Learning Networks
Sep 10, 2017



Abstract:Deep learning has become a promising approach for automated medical diagnoses. When medical data samples are limited, collaboration among multiple institutions is necessary to achieve high algorithm performance. However, sharing patient data often has limitations due to technical, legal, or ethical concerns. In such cases, sharing a deep learning model is a more attractive alternative. The best method of performing such a task is unclear, however. In this study, we simulate the dissemination of learning deep learning network models across four institutions using various heuristics and compare the results with a deep learning model trained on centrally hosted patient data. The heuristics investigated include ensembling single institution models, single weight transfer, and cyclical weight transfer. We evaluated these approaches for image classification in three independent image collections (retinal fundus photos, mammography, and ImageNet). We find that cyclical weight transfer resulted in a performance (testing accuracy = 77.3%) that was closest to that of centrally hosted patient data (testing accuracy = 78.7%). We also found that there is an improvement in the performance of cyclical weight transfer heuristic with high frequency of weight transfer.
Sequential 3D U-Nets for Biologically-Informed Brain Tumor Segmentation
Sep 09, 2017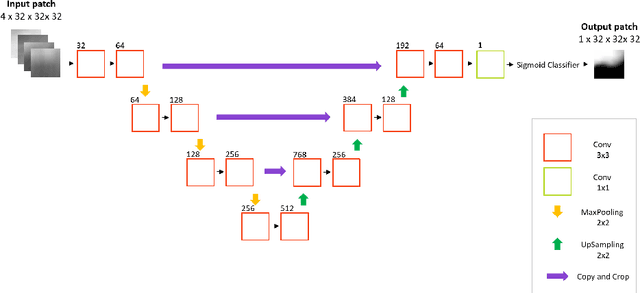

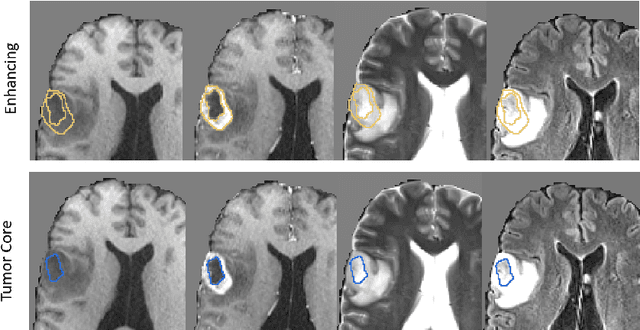
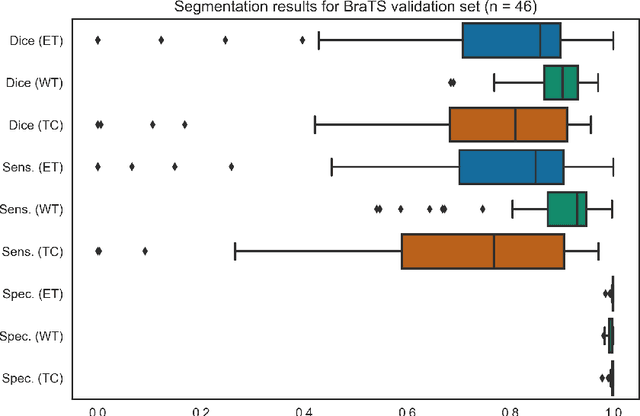
Abstract:Deep learning has quickly become the weapon of choice for brain lesion segmentation. However, few existing algorithms pre-configure any biological context of their chosen segmentation tissues, and instead rely on the neural network's optimizer to develop such associations de novo. We present a novel method for applying deep neural networks to the problem of glioma tissue segmentation that takes into account the structured nature of gliomas - edematous tissue surrounding mutually-exclusive regions of enhancing and non-enhancing tumor. We trained multiple deep neural networks with a 3D U-Net architecture in a tree structure to create segmentations for edema, non-enhancing tumor, and enhancing tumor regions. Specifically, training was configured such that the whole tumor region including edema was predicted first, and its output segmentation was fed as input into separate models to predict enhancing and non-enhancing tumor. Our method was trained and evaluated on the publicly available BraTS dataset, achieving Dice scores of 0.882, 0.732, and 0.730 for whole tumor, enhancing tumor and tumor core respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge