André Ferreira
Centro Algoritmi, University of Minho, Braga, Portugal
Curated endoscopic retrograde cholangiopancreatography images dataset
Jan 23, 2026Abstract:Endoscopic Retrograde Cholangiopancreatography (ERCP) is a key procedure in the diagnosis and treatment of biliary and pancreatic diseases. Artificial intelligence has been pointed as one solution to automatize diagnosis. However, public ERCP datasets are scarce, which limits the use of such approach. Therefore, this study aims to help fill this gap by providing a large and curated dataset. The collection is composed of 19.018 raw images and 19.317 processed from 1.602 patients. 5.519 images are labeled, which provides a ready to use dataset. All images were manually inspected and annotated by two gastroenterologist with more than 5 years of experience and reviewed by another gastroenterologist with more than 20 years of experience, all with more than 400 ERCP procedures annually. The utility and validity of the dataset is proven by a classification experiment. This collection aims to provide or contribute for a benchmark in automatic ERCP analysis and diagnosis of biliary and pancreatic diseases.
Enhancing Privacy: The Utility of Stand-Alone Synthetic CT and MRI for Tumor and Bone Segmentation
Jun 13, 2025Abstract:AI requires extensive datasets, while medical data is subject to high data protection. Anonymization is essential, but poses a challenge for some regions, such as the head, as identifying structures overlap with regions of clinical interest. Synthetic data offers a potential solution, but studies often lack rigorous evaluation of realism and utility. Therefore, we investigate to what extent synthetic data can replace real data in segmentation tasks. We employed head and neck cancer CT scans and brain glioma MRI scans from two large datasets. Synthetic data were generated using generative adversarial networks and diffusion models. We evaluated the quality of the synthetic data using MAE, MS-SSIM, Radiomics and a Visual Turing Test (VTT) performed by 5 radiologists and their usefulness in segmentation tasks using DSC. Radiomics indicates high fidelity of synthetic MRIs, but fall short in producing highly realistic CT tissue, with correlation coefficient of 0.8784 and 0.5461 for MRI and CT tumors, respectively. DSC results indicate limited utility of synthetic data: tumor segmentation achieved DSC=0.064 on CT and 0.834 on MRI, while bone segmentation a mean DSC=0.841. Relation between DSC and correlation is observed, but is limited by the complexity of the task. VTT results show synthetic CTs' utility, but with limited educational applications. Synthetic data can be used independently for the segmentation task, although limited by the complexity of the structures to segment. Advancing generative models to better tolerate heterogeneous inputs and learn subtle details is essential for enhancing their realism and expanding their application potential.
Comparative Analysis of nnUNet and MedNeXt for Head and Neck Tumor Segmentation in MRI-guided Radiotherapy
Nov 22, 2024Abstract:Radiation therapy (RT) is essential in treating head and neck cancer (HNC), with magnetic resonance imaging(MRI)-guided RT offering superior soft tissue contrast and functional imaging. However, manual tumor segmentation is time-consuming and complex, and therfore remains a challenge. In this study, we present our solution as team TUMOR to the HNTS-MRG24 MICCAI Challenge which is focused on automated segmentation of primary gross tumor volumes (GTVp) and metastatic lymph node gross tumor volume (GTVn) in pre-RT and mid-RT MRI images. We utilized the HNTS-MRG2024 dataset, which consists of 150 MRI scans from patients diagnosed with HNC, including original and registered pre-RT and mid-RT T2-weighted images with corresponding segmentation masks for GTVp and GTVn. We employed two state-of-the-art models in deep learning, nnUNet and MedNeXt. For Task 1, we pretrained models on pre-RT registered and mid-RT images, followed by fine-tuning on original pre-RT images. For Task 2, we combined registered pre-RT images, registered pre-RT segmentation masks, and mid-RT data as a multi-channel input for training. Our solution for Task 1 achieved 1st place in the final test phase with an aggregated Dice Similarity Coefficient of 0.8254, and our solution for Task 2 ranked 8th with a score of 0.7005. The proposed solution is publicly available at Github Repository.
Improved Multi-Task Brain Tumour Segmentation with Synthetic Data Augmentation
Nov 07, 2024



Abstract:This paper presents the winning solution of task 1 and the third-placed solution of task 3 of the BraTS challenge. The use of automated tools in clinical practice has increased due to the development of more and more sophisticated and reliable algorithms. However, achieving clinical standards and developing tools for real-life scenarios is a major challenge. To this end, BraTS has organised tasks to find the most advanced solutions for specific purposes. In this paper, we propose the use of synthetic data to train state-of-the-art frameworks in order to improve the segmentation of adult gliomas in a post-treatment scenario, and the segmentation of meningioma for radiotherapy planning. Our results suggest that the use of synthetic data leads to more robust algorithms, although the synthetic data generation pipeline is not directly suited to the meningioma task. The code for these tasks is available at https://github.com/ShadowTwin41/BraTS_2023_2024_solutions.
Brain Tumour Removing and Missing Modality Generation using 3D WDM
Nov 07, 2024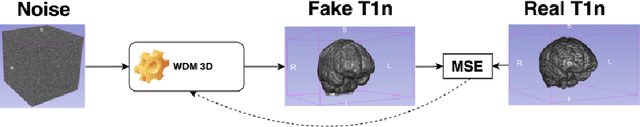
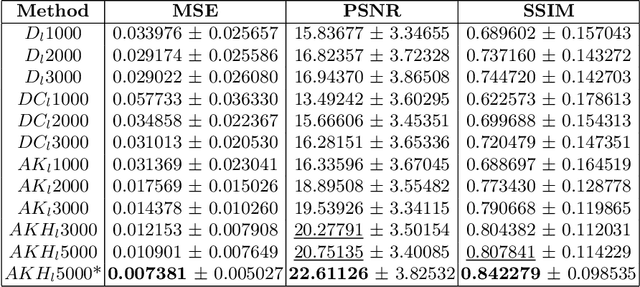
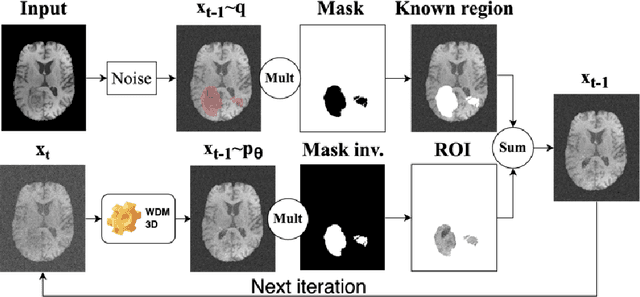
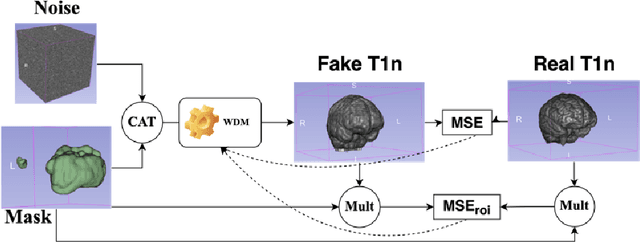
Abstract:This paper presents the second-placed solution for task 8 and the participation solution for task 7 of BraTS 2024. The adoption of automated brain analysis algorithms to support clinical practice is increasing. However, many of these algorithms struggle with the presence of brain lesions or the absence of certain MRI modalities. The alterations in the brain's morphology leads to high variability and thus poor performance of predictive models that were trained only on healthy brains. The lack of information that is usually provided by some of the missing MRI modalities also reduces the reliability of the prediction models trained with all modalities. In order to improve the performance of these models, we propose the use of conditional 3D wavelet diffusion models. The wavelet transform enabled full-resolution image training and prediction on a GPU with 48 GB VRAM, without patching or downsampling, preserving all information for prediction. For the inpainting task of BraTS 2024, the use of a large and variable number of healthy masks and the stability and efficiency of the 3D wavelet diffusion model resulted in 0.007, 22.61 and 0.842 in the validation set and 0.07 , 22.8 and 0.91 in the testing set (MSE, PSNR and SSIM respectively). The code for these tasks is available at https://github.com/ShadowTwin41/BraTS_2023_2024_solutions.
Deep Dive into MRI: Exploring Deep Learning Applications in 0.55T and 7T MRI
Jul 01, 2024



Abstract:The development of magnetic resonance imaging (MRI) for medical imaging has provided a leap forward in diagnosis, providing a safe, non-invasive alternative to techniques involving ionising radiation exposure for diagnostic purposes. It was described by Block and Purcel in 1946, and it was not until 1980 that the first clinical application of MRI became available. Since that time the MRI has gone through many advances and has altered the way diagnosing procedures are performed. Due to its ability to improve constantly, MRI has become a commonly used practice among several specialisations in medicine. Particularly starting 0.55T and 7T MRI technologies have pointed out enhanced preservation of image detail and advanced tissue characterisation. This review examines the integration of deep learning (DL) techniques into these MRI modalities, disseminating and exploring the study applications. It highlights how DL contributes to 0.55T and 7T MRI data, showcasing the potential of DL in improving and refining these technologies. The review ends with a brief overview of how MRI technology will evolve in the coming years.
How we won BraTS 2023 Adult Glioma challenge? Just faking it! Enhanced Synthetic Data Augmentation and Model Ensemble for brain tumour segmentation
Feb 27, 2024Abstract:Deep Learning is the state-of-the-art technology for segmenting brain tumours. However, this requires a lot of high-quality data, which is difficult to obtain, especially in the medical field. Therefore, our solutions address this problem by using unconventional mechanisms for data augmentation. Generative adversarial networks and registration are used to massively increase the amount of available samples for training three different deep learning models for brain tumour segmentation, the first task of the BraTS2023 challenge. The first model is the standard nnU-Net, the second is the Swin UNETR and the third is the winning solution of the BraTS 2021 Challenge. The entire pipeline is built on the nnU-Net implementation, except for the generation of the synthetic data. The use of convolutional algorithms and transformers is able to fill each other's knowledge gaps. Using the new metric, our best solution achieves the dice results 0.9005, 0.8673, 0.8509 and HD95 14.940, 14.467, 17.699 (whole tumour, tumour core and enhancing tumour) in the validation set.
Deep PCCT: Photon Counting Computed Tomography Deep Learning Applications Review
Feb 06, 2024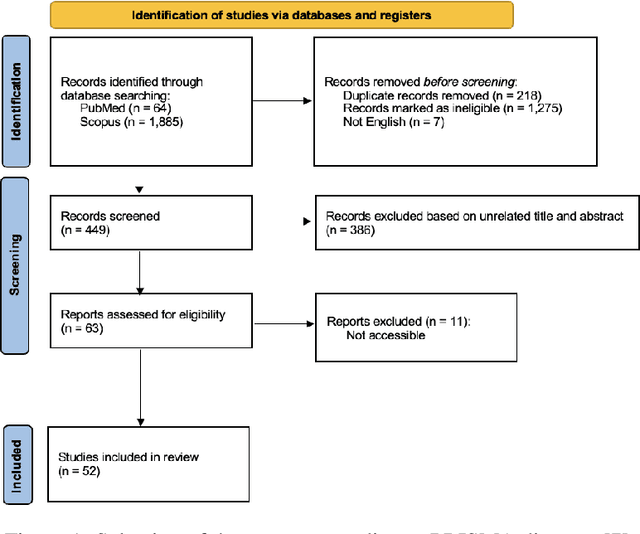
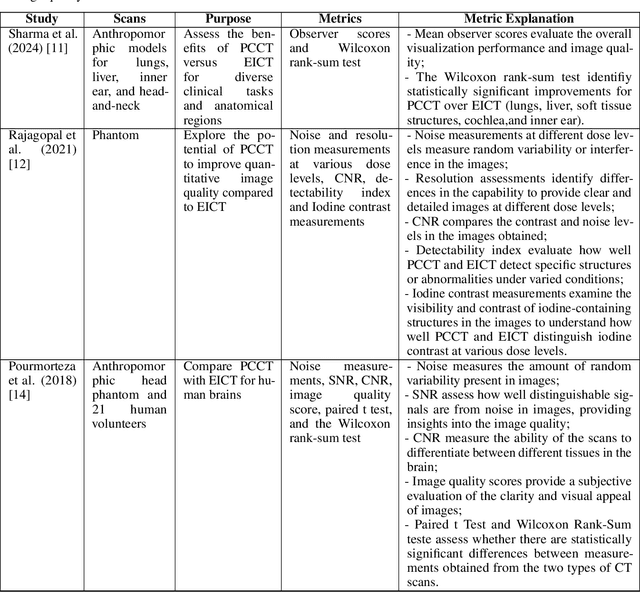
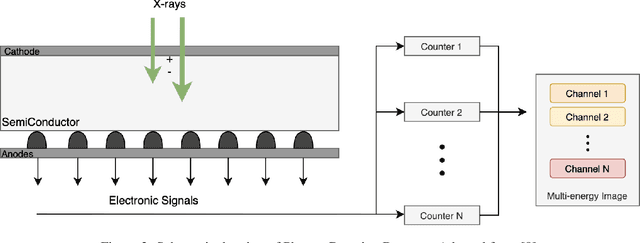
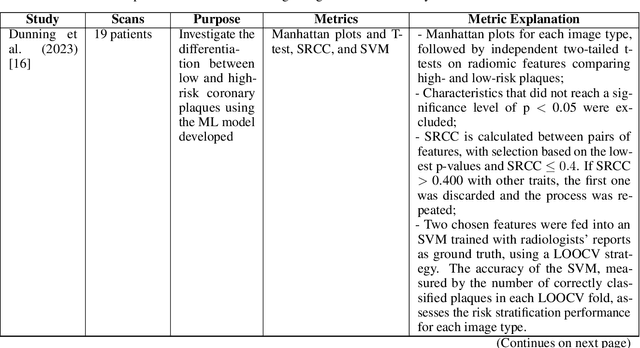
Abstract:Medical imaging faces challenges such as limited spatial resolution, interference from electronic noise and poor contrast-to-noise ratios. Photon Counting Computed Tomography (PCCT) has emerged as a solution, addressing these issues with its innovative technology. This review delves into the recent developments and applications of PCCT in pre-clinical research, emphasizing its potential to overcome traditional imaging limitations. For example PCCT has demonstrated remarkable efficacy in improving the detection of subtle abnormalities in breast, providing a level of detail previously unattainable. Examining the current literature on PCCT, it presents a comprehensive analysis of the technology, highlighting the main features of scanners and their varied applications. In addition, it explores the integration of deep learning into PCCT, along with the study of radiomic features, presenting successful applications in data processing. While acknowledging these advances, it also discusses the existing challenges in this field, paving the way for future research and improvements in medical imaging technologies. Despite the limited number of articles on this subject, due to the recent integration of PCCT at a clinical level, its potential benefits extend to various diagnostic applications.
Multilingual Natural Language Processing Model for Radiology Reports -- The Summary is all you need!
Oct 06, 2023



Abstract:The impression section of a radiology report summarizes important radiology findings and plays a critical role in communicating these findings to physicians. However, the preparation of these summaries is time-consuming and error-prone for radiologists. Recently, numerous models for radiology report summarization have been developed. Nevertheless, there is currently no model that can summarize these reports in multiple languages. Such a model could greatly improve future research and the development of Deep Learning models that incorporate data from patients with different ethnic backgrounds. In this study, the generation of radiology impressions in different languages was automated by fine-tuning a model, publicly available, based on a multilingual text-to-text Transformer to summarize findings available in English, Portuguese, and German radiology reports. In a blind test, two board-certified radiologists indicated that for at least 70% of the system-generated summaries, the quality matched or exceeded the corresponding human-written summaries, suggesting substantial clinical reliability. Furthermore, this study showed that the multilingual model outperformed other models that specialized in summarizing radiology reports in only one language, as well as models that were not specifically designed for summarizing radiology reports, such as ChatGPT.
MedShapeNet -- A Large-Scale Dataset of 3D Medical Shapes for Computer Vision
Sep 12, 2023



Abstract:We present MedShapeNet, a large collection of anatomical shapes (e.g., bones, organs, vessels) and 3D surgical instrument models. Prior to the deep learning era, the broad application of statistical shape models (SSMs) in medical image analysis is evidence that shapes have been commonly used to describe medical data. Nowadays, however, state-of-the-art (SOTA) deep learning algorithms in medical imaging are predominantly voxel-based. In computer vision, on the contrary, shapes (including, voxel occupancy grids, meshes, point clouds and implicit surface models) are preferred data representations in 3D, as seen from the numerous shape-related publications in premier vision conferences, such as the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), as well as the increasing popularity of ShapeNet (about 51,300 models) and Princeton ModelNet (127,915 models) in computer vision research. MedShapeNet is created as an alternative to these commonly used shape benchmarks to facilitate the translation of data-driven vision algorithms to medical applications, and it extends the opportunities to adapt SOTA vision algorithms to solve critical medical problems. Besides, the majority of the medical shapes in MedShapeNet are modeled directly on the imaging data of real patients, and therefore it complements well existing shape benchmarks comprising of computer-aided design (CAD) models. MedShapeNet currently includes more than 100,000 medical shapes, and provides annotations in the form of paired data. It is therefore also a freely available repository of 3D models for extended reality (virtual reality - VR, augmented reality - AR, mixed reality - MR) and medical 3D printing. This white paper describes in detail the motivations behind MedShapeNet, the shape acquisition procedures, the use cases, as well as the usage of the online shape search portal: https://medshapenet.ikim.nrw/
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge