Christina Gsaxner
Efficient MedSAMs: Segment Anything in Medical Images on Laptop
Dec 20, 2024


Abstract:Promptable segmentation foundation models have emerged as a transformative approach to addressing the diverse needs in medical images, but most existing models require expensive computing, posing a big barrier to their adoption in clinical practice. In this work, we organized the first international competition dedicated to promptable medical image segmentation, featuring a large-scale dataset spanning nine common imaging modalities from over 20 different institutions. The top teams developed lightweight segmentation foundation models and implemented an efficient inference pipeline that substantially reduced computational requirements while maintaining state-of-the-art segmentation accuracy. Moreover, the post-challenge phase advanced the algorithms through the design of performance booster and reproducibility tasks, resulting in improved algorithms and validated reproducibility of the winning solution. Furthermore, the best-performing algorithms have been incorporated into the open-source software with a user-friendly interface to facilitate clinical adoption. The data and code are publicly available to foster the further development of medical image segmentation foundation models and pave the way for impactful real-world applications.
Deep Learning-based Point Cloud Registration for Augmented Reality-guided Surgery
May 06, 2024Abstract:Point cloud registration aligns 3D point clouds using spatial transformations. It is an important task in computer vision, with applications in areas such as augmented reality (AR) and medical imaging. This work explores the intersection of two research trends: the integration of AR into image-guided surgery and the use of deep learning for point cloud registration. The main objective is to evaluate the feasibility of applying deep learning-based point cloud registration methods for image-to-patient registration in augmented reality-guided surgery. We created a dataset of point clouds from medical imaging and corresponding point clouds captured with a popular AR device, the HoloLens 2. We evaluate three well-established deep learning models in registering these data pairs. While we find that some deep learning methods show promise, we show that a conventional registration pipeline still outperforms them on our challenging dataset.
Deep Medial Voxels: Learned Medial Axis Approximations for Anatomical Shape Modeling
Mar 18, 2024Abstract:Shape reconstruction from imaging volumes is a recurring need in medical image analysis. Common workflows start with a segmentation step, followed by careful post-processing and,finally, ad hoc meshing algorithms. As this sequence can be timeconsuming, neural networks are trained to reconstruct shapes through template deformation. These networks deliver state-ofthe-art results without manual intervention, but, so far, they have primarily been evaluated on anatomical shapes with little topological variety between individuals. In contrast, other works favor learning implicit shape models, which have multiple benefits for meshing and visualization. Our work follows this direction by introducing deep medial voxels, a semi-implicit representation that faithfully approximates the topological skeleton from imaging volumes and eventually leads to shape reconstruction via convolution surfaces. Our reconstruction technique shows potential for both visualization and computer simulations.
DeepDR: Deep Structure-Aware RGB-D Inpainting for Diminished Reality
Dec 01, 2023Abstract:Diminished reality (DR) refers to the removal of real objects from the environment by virtually replacing them with their background. Modern DR frameworks use inpainting to hallucinate unobserved regions. While recent deep learning-based inpainting is promising, the DR use case is complicated by the need to generate coherent structure and 3D geometry (i.e., depth), in particular for advanced applications, such as 3D scene editing. In this paper, we propose DeepDR, a first RGB-D inpainting framework fulfilling all requirements of DR: Plausible image and geometry inpainting with coherent structure, running at real-time frame rates, with minimal temporal artifacts. Our structure-aware generative network allows us to explicitly condition color and depth outputs on the scene semantics, overcoming the difficulty of reconstructing sharp and consistent boundaries in regions with complex backgrounds. Experimental results show that the proposed framework can outperform related work qualitatively and quantitatively.
MedShapeNet -- A Large-Scale Dataset of 3D Medical Shapes for Computer Vision
Sep 12, 2023



Abstract:We present MedShapeNet, a large collection of anatomical shapes (e.g., bones, organs, vessels) and 3D surgical instrument models. Prior to the deep learning era, the broad application of statistical shape models (SSMs) in medical image analysis is evidence that shapes have been commonly used to describe medical data. Nowadays, however, state-of-the-art (SOTA) deep learning algorithms in medical imaging are predominantly voxel-based. In computer vision, on the contrary, shapes (including, voxel occupancy grids, meshes, point clouds and implicit surface models) are preferred data representations in 3D, as seen from the numerous shape-related publications in premier vision conferences, such as the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), as well as the increasing popularity of ShapeNet (about 51,300 models) and Princeton ModelNet (127,915 models) in computer vision research. MedShapeNet is created as an alternative to these commonly used shape benchmarks to facilitate the translation of data-driven vision algorithms to medical applications, and it extends the opportunities to adapt SOTA vision algorithms to solve critical medical problems. Besides, the majority of the medical shapes in MedShapeNet are modeled directly on the imaging data of real patients, and therefore it complements well existing shape benchmarks comprising of computer-aided design (CAD) models. MedShapeNet currently includes more than 100,000 medical shapes, and provides annotations in the form of paired data. It is therefore also a freely available repository of 3D models for extended reality (virtual reality - VR, augmented reality - AR, mixed reality - MR) and medical 3D printing. This white paper describes in detail the motivations behind MedShapeNet, the shape acquisition procedures, the use cases, as well as the usage of the online shape search portal: https://medshapenet.ikim.nrw/
Apple Vision Pro for Healthcare: "The Ultimate Display"? -- Entering the Wonderland of Precision
Aug 10, 2023Abstract:At the Worldwide Developers Conference (WWDC) in June 2023, Apple introduced the Vision Pro. The Vision Pro is a Mixed Reality (MR) headset, more specifically it is a Virtual Reality (VR) device with an additional Video See-Through (VST) capability. The VST capability turns the Vision Pro also into an Augmented Reality (AR) device. The AR feature is enabled by streaming the real world via cameras to the (VR) screens in front of the user's eyes. This is of course not unique and similar to other devices, like the Varjo XR-3. Nevertheless, the Vision Pro has some interesting features, like an inside-out screen that can show the headset wearers' eyes to "outsiders" or a button on the top, called "Digital Crown", that allows you to seamlessly blend digital content with your physical space by turning it. In addition, it is untethered, except for the cable to the battery, which makes the headset more agile, compared to the Varjo XR-3. This could actually come closer to the "Ultimate Display", which Ivan Sutherland had already sketched in 1965. Not available to the public yet, like the Ultimate Display, we want to take a look into the crystal ball in this perspective to see if it can overcome some clinical challenges that - especially - AR still faces in the medical domain, but also go beyond and discuss if the Vision Pro could support clinicians in essential tasks to spend more time with their patients.
The HoloLens in Medicine: A systematic Review and Taxonomy
Sep 06, 2022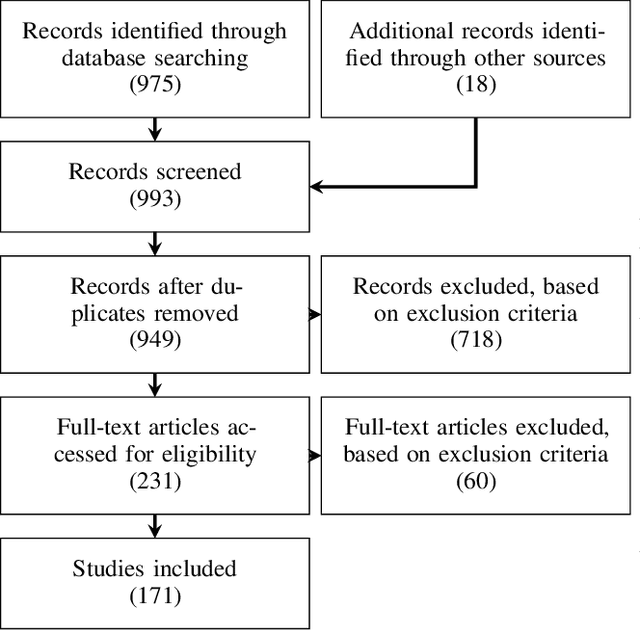
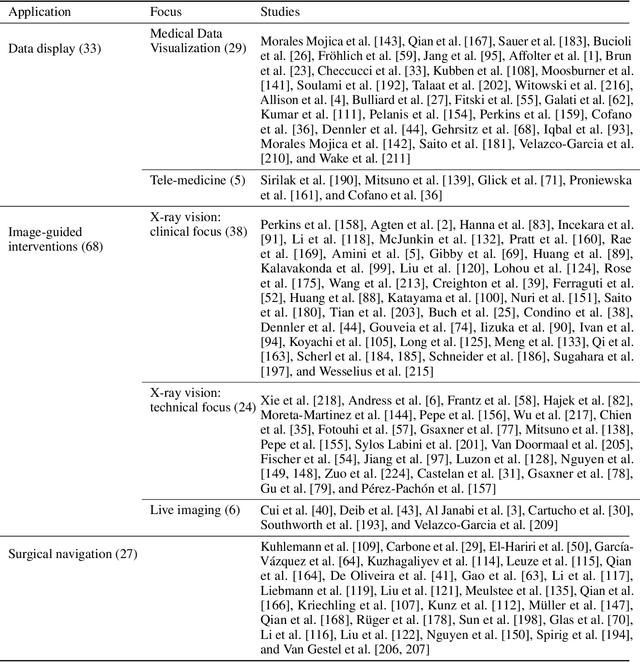
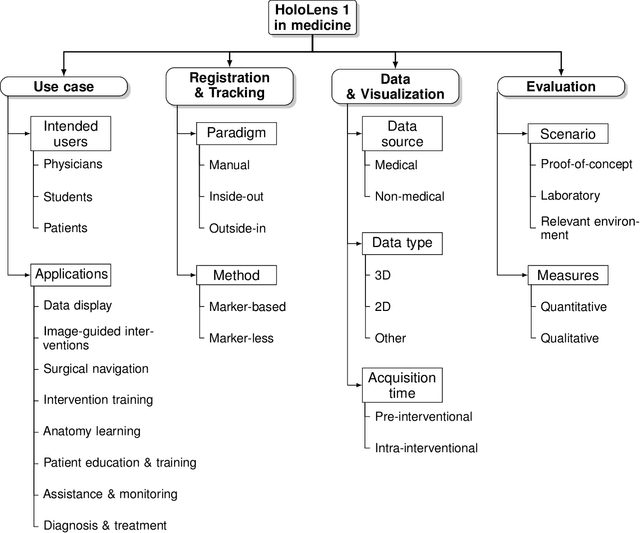

Abstract:The HoloLens (Microsoft Corp., Redmond, WA), a head-worn, optically see-through augmented reality display, is the main player in the recent boost in medical augmented reality research. In medical settings, the HoloLens enables the physician to obtain immediate insight into patient information, directly overlaid with their view of the clinical scenario, the medical student to gain a better understanding of complex anatomies or procedures, and even the patient to execute therapeutic tasks with improved, immersive guidance. In this systematic review, we provide a comprehensive overview of the usage of the first-generation HoloLens within the medical domain, from its release in March 2016, until the year of 2021, were attention is shifting towards it's successor, the HoloLens 2. We identified 171 relevant publications through a systematic search of the PubMed and Scopus databases. We analyze these publications in regard to their intended use case, technical methodology for registration and tracking, data sources, visualization as well as validation and evaluation. We find that, although the feasibility of using the HoloLens in various medical scenarios has been shown, increased efforts in the areas of precision, reliability, usability, workflow and perception are necessary to establish AR in clinical practice.
Back to the Roots: Reconstructing Large and Complex Cranial Defects using an Image-based Statistical Shape Model
Apr 12, 2022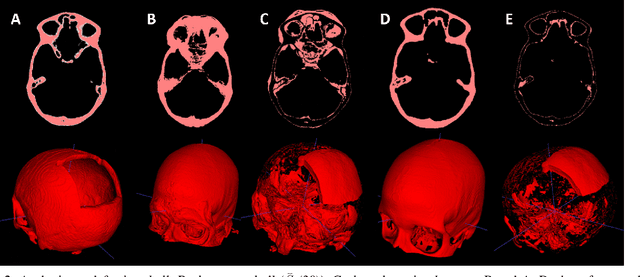
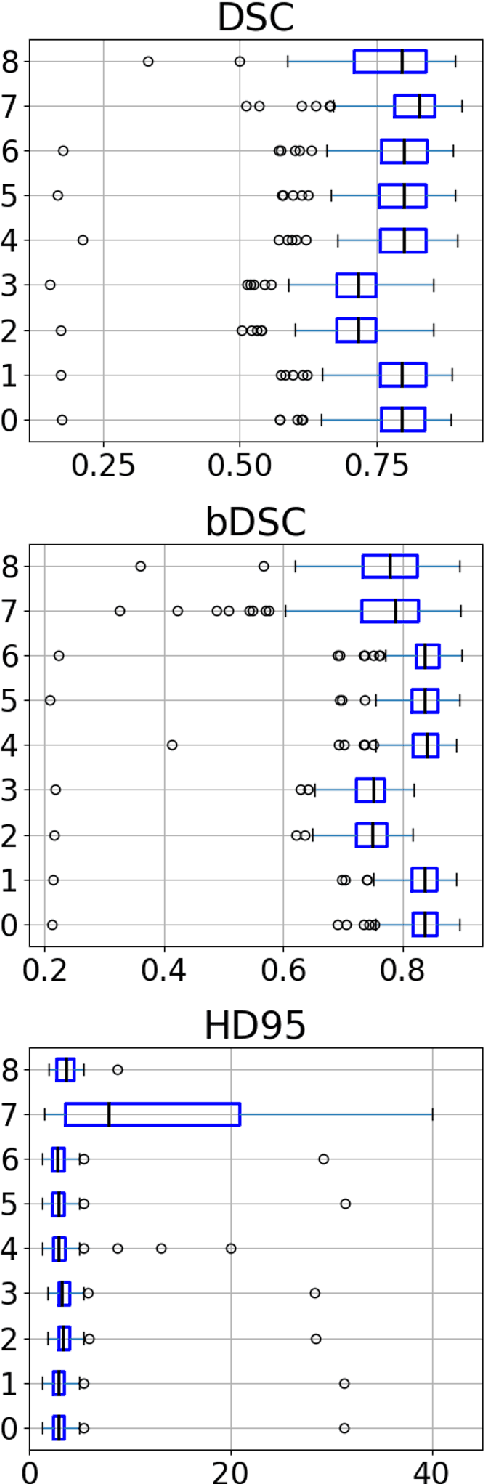
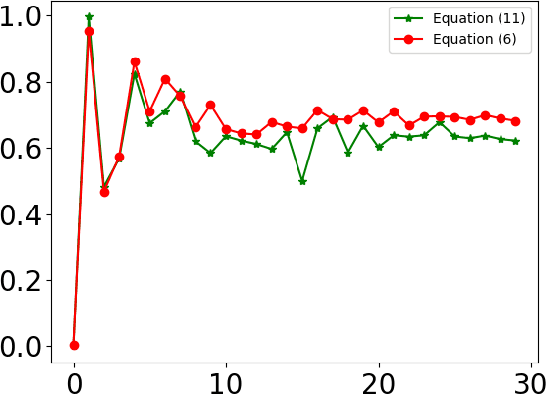
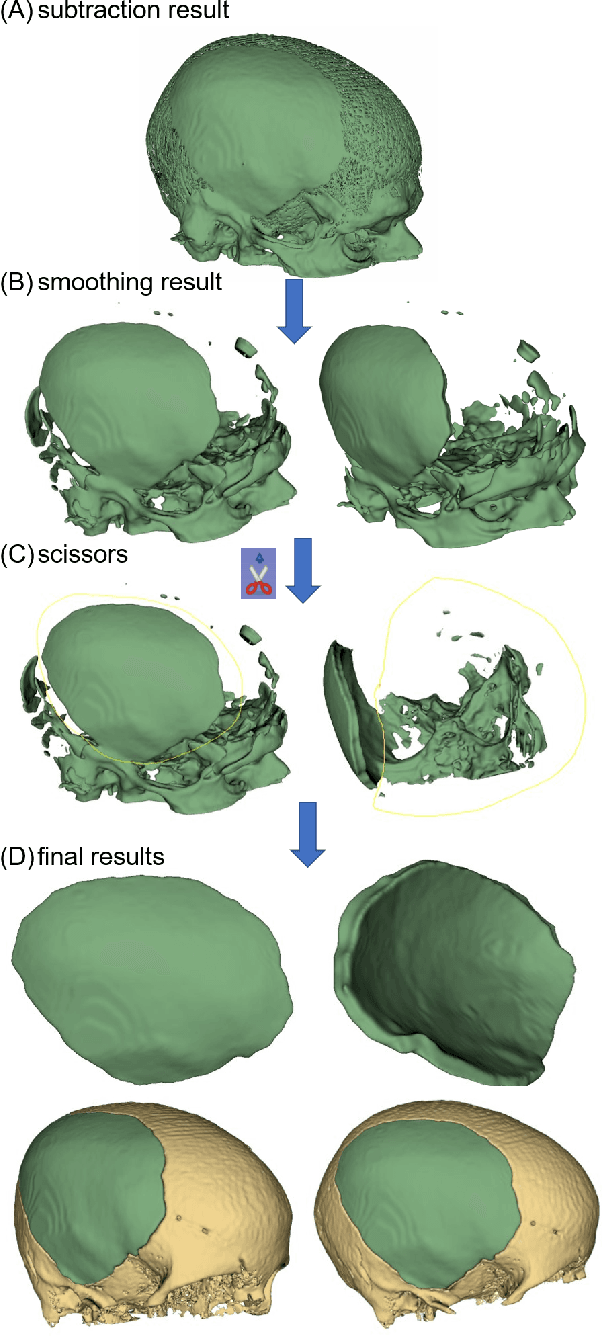
Abstract:Designing implants for large and complex cranial defects is a challenging task, even for professional designers. Current efforts on automating the design process focused mainly on convolutional neural networks (CNN), which have produced state-of-the-art results on reconstructing synthetic defects. However, existing CNN-based methods have been difficult to translate to clinical practice in cranioplasty, as their performance on complex and irregular cranial defects remains unsatisfactory. In this paper, a statistical shape model (SSM) built directly on the segmentation masks of the skulls is presented. We evaluate the SSM on several cranial implant design tasks, and the results show that, while the SSM performs suboptimally on synthetic defects compared to CNN-based approaches, it is capable of reconstructing large and complex defects with only minor manual corrections. The quality of the resulting implants is examined and assured by experienced neurosurgeons. In contrast, CNN-based approaches, even with massive data augmentation, fail or produce less-than-satisfactory implants for these cases. Codes are publicly available at https://github.com/Jianningli/ssm
Automated cross-sectional view selection in CT angiography of aortic dissections with uncertainty awareness and retrospective clinical annotations
Nov 22, 2021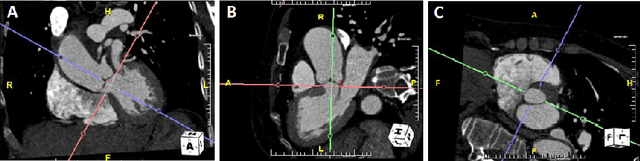
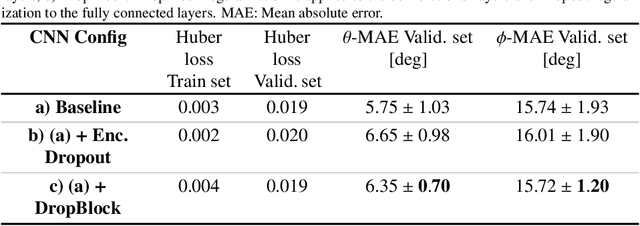
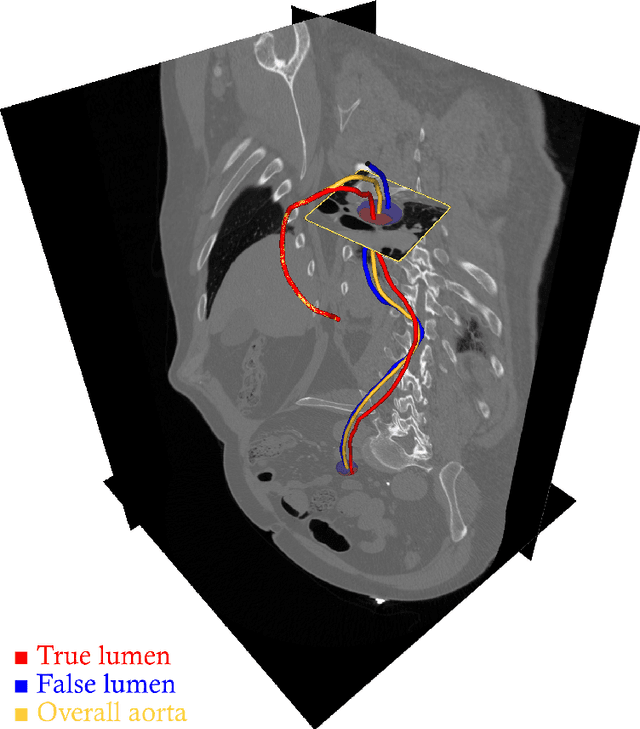
Abstract:Objective: Surveillance imaging of chronic aortic diseases, such as dissections, relies on obtaining and comparing cross-sectional diameter measurements at predefined aortic landmarks, over time. Due to a lack of robust tools, the orientation of the cross-sectional planes is defined manually by highly trained operators. We show how manual annotations routinely collected in a clinic can be efficiently used to ease this task, despite the presence of a non-negligible interoperator variability in the measurements. Impact: Ill-posed but repetitive imaging tasks can be eased or automated by leveraging imperfect, retrospective clinical annotations. Methodology: In this work, we combine convolutional neural networks and uncertainty quantification methods to predict the orientation of such cross-sectional planes. We use clinical data randomly processed by 11 operators for training, and test on a smaller set processed by 3 independent operators to assess interoperator variability. Results: Our analysis shows that manual selection of cross-sectional planes is characterized by 95% limits of agreement (LOA) of $10.6^\circ$ and $21.4^\circ$ per angle. Our method showed to decrease static error by $3.57^\circ$ ($40.2$%) and $4.11^\circ$ ($32.8$%) against state of the art and LOA by $5.4^\circ$ ($49.0$%) and $16.0^\circ$ ($74.6$%) against manual processing. Conclusion: This suggests that pre-existing annotations can be an inexpensive resource in clinics to ease ill-posed and repetitive tasks like cross-section extraction for surveillance of aortic dissections.
Learning to Rearrange Voxels in Binary Segmentation Masks for Smooth Manifold Triangulation
Aug 11, 2021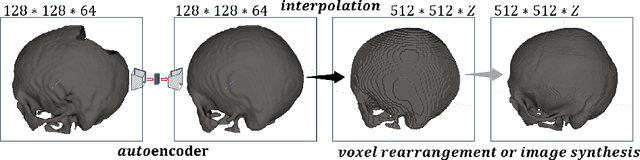
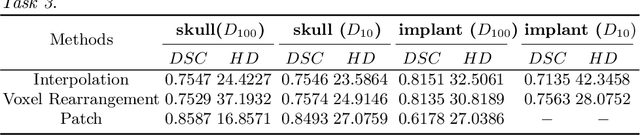
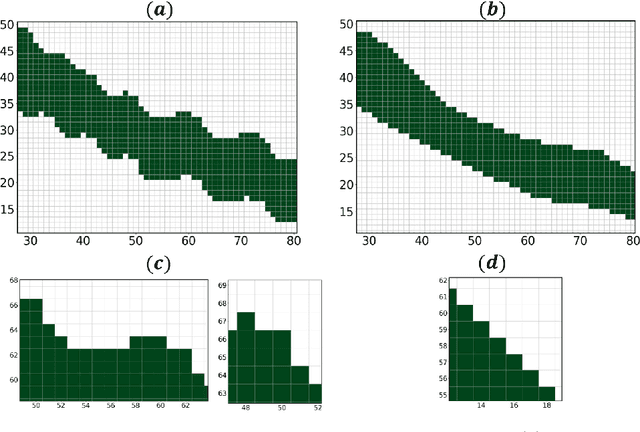
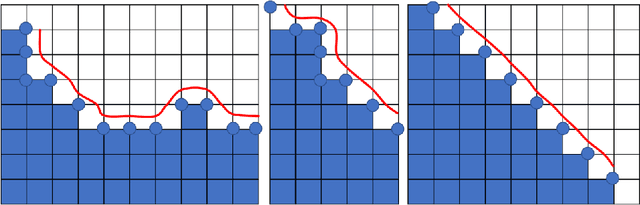
Abstract:Medical images, especially volumetric images, are of high resolution and often exceed the capacity of standard desktop GPUs. As a result, most deep learning-based medical image analysis tasks require the input images to be downsampled, often substantially, before these can be fed to a neural network. However, downsampling can lead to a loss of image quality, which is undesirable especially in reconstruction tasks, where the fine geometric details need to be preserved. In this paper, we propose that high-resolution images can be reconstructed in a coarse-to-fine fashion, where a deep learning algorithm is only responsible for generating a coarse representation of the image, which consumes moderate GPU memory. For producing the high-resolution outcome, we propose two novel methods: learned voxel rearrangement of the coarse output and hierarchical image synthesis. Compared to the coarse output, the high-resolution counterpart allows for smooth surface triangulation, which can be 3D-printed in the highest possible quality. Experiments of this paper are carried out on the dataset of AutoImplant 2021 (https://autoimplant2021.grand-challenge.org/), a MICCAI challenge on cranial implant design. The dataset contains high-resolution skulls that can be viewed as 2D manifolds embedded in a 3D space. Codes associated with this study can be accessed at https://github.com/Jianningli/voxel_rearrangement.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge