Naida Solak
How we won BraTS 2023 Adult Glioma challenge? Just faking it! Enhanced Synthetic Data Augmentation and Model Ensemble for brain tumour segmentation
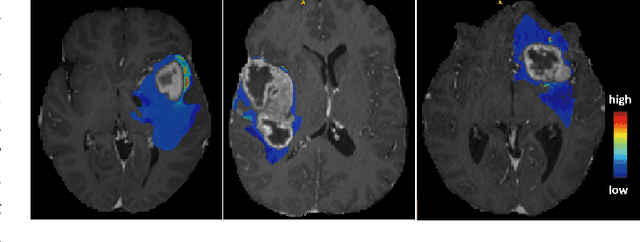
Feb 27, 2024Abstract:Deep Learning is the state-of-the-art technology for segmenting brain tumours. However, this requires a lot of high-quality data, which is difficult to obtain, especially in the medical field. Therefore, our solutions address this problem by using unconventional mechanisms for data augmentation. Generative adversarial networks and registration are used to massively increase the amount of available samples for training three different deep learning models for brain tumour segmentation, the first task of the BraTS2023 challenge. The first model is the standard nnU-Net, the second is the Swin UNETR and the third is the winning solution of the BraTS 2021 Challenge. The entire pipeline is built on the nnU-Net implementation, except for the generation of the synthetic data. The use of convolutional algorithms and transformers is able to fill each other's knowledge gaps. Using the new metric, our best solution achieves the dice results 0.9005, 0.8673, 0.8509 and HD95 14.940, 14.467, 17.699 (whole tumour, tumour core and enhancing tumour) in the validation set.
MedShapeNet -- A Large-Scale Dataset of 3D Medical Shapes for Computer Vision
Sep 12, 2023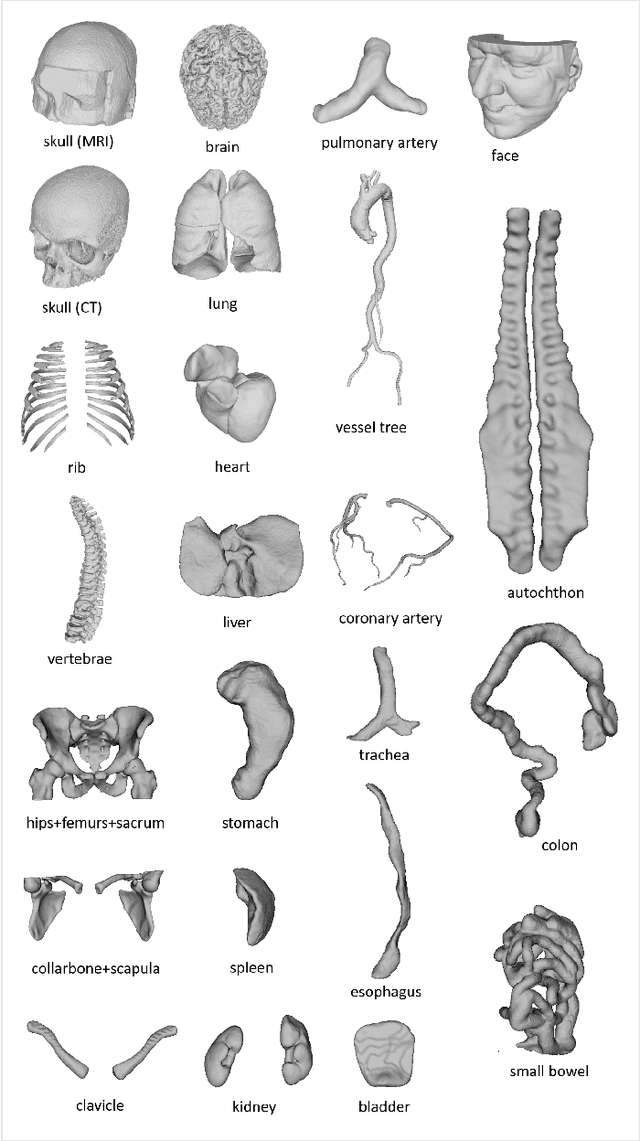

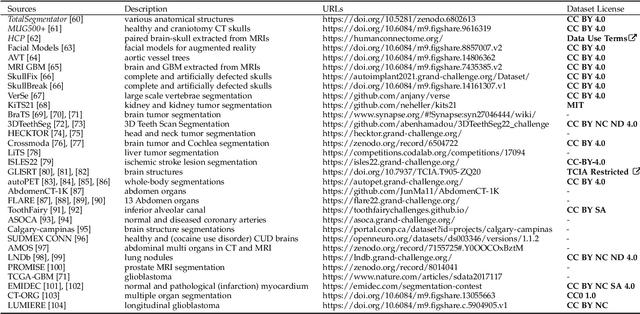
Abstract:We present MedShapeNet, a large collection of anatomical shapes (e.g., bones, organs, vessels) and 3D surgical instrument models. Prior to the deep learning era, the broad application of statistical shape models (SSMs) in medical image analysis is evidence that shapes have been commonly used to describe medical data. Nowadays, however, state-of-the-art (SOTA) deep learning algorithms in medical imaging are predominantly voxel-based. In computer vision, on the contrary, shapes (including, voxel occupancy grids, meshes, point clouds and implicit surface models) are preferred data representations in 3D, as seen from the numerous shape-related publications in premier vision conferences, such as the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), as well as the increasing popularity of ShapeNet (about 51,300 models) and Princeton ModelNet (127,915 models) in computer vision research. MedShapeNet is created as an alternative to these commonly used shape benchmarks to facilitate the translation of data-driven vision algorithms to medical applications, and it extends the opportunities to adapt SOTA vision algorithms to solve critical medical problems. Besides, the majority of the medical shapes in MedShapeNet are modeled directly on the imaging data of real patients, and therefore it complements well existing shape benchmarks comprising of computer-aided design (CAD) models. MedShapeNet currently includes more than 100,000 medical shapes, and provides annotations in the form of paired data. It is therefore also a freely available repository of 3D models for extended reality (virtual reality - VR, augmented reality - AR, mixed reality - MR) and medical 3D printing. This white paper describes in detail the motivations behind MedShapeNet, the shape acquisition procedures, the use cases, as well as the usage of the online shape search portal: https://medshapenet.ikim.nrw/
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge