Yujin Oh
CoMMa: Contribution-Aware Medical Multi-Agents From A Game-Theoretic Perspective
Feb 09, 2026Abstract:Recent multi-agent frameworks have broadened the ability to tackle oncology decision support tasks that require reasoning over dynamic, heterogeneous patient data. We propose Contribution-Aware Medical Multi-Agents (CoMMa), a decentralized LLM-agent framework in which specialists operate on partitioned evidence and coordinate through a game-theoretic objective for robust decision-making. In contrast to most agent architectures relying on stochastic narrative-based reasoning, CoMMa utilizes deterministic embedding projections to approximate contribution-aware credit assignment. This yields explicit evidence attribution by estimating each agent's marginal utility, producing interpretable and mathematically grounded decision pathways with improved stability. Evaluated on diverse oncology benchmarks, including a real-world multidisciplinary tumor board dataset, CoMMa achieves higher accuracy and more stable performance than data-centralized and role-based multi-agents baselines.
Surf2CT: Cascaded 3D Flow Matching Models for Torso 3D CT Synthesis from Skin Surface
May 29, 2025Abstract:We present Surf2CT, a novel cascaded flow matching framework that synthesizes full 3D computed tomography (CT) volumes of the human torso from external surface scans and simple demographic data (age, sex, height, weight). This is the first approach capable of generating realistic volumetric internal anatomy images solely based on external body shape and demographics, without any internal imaging. Surf2CT proceeds through three sequential stages: (1) Surface Completion, reconstructing a complete signed distance function (SDF) from partial torso scans using conditional 3D flow matching; (2) Coarse CT Synthesis, generating a low-resolution CT volume from the completed SDF and demographic information; and (3) CT Super-Resolution, refining the coarse volume into a high-resolution CT via a patch-wise conditional flow model. Each stage utilizes a 3D-adapted EDM2 backbone trained via flow matching. We trained our model on a combined dataset of 3,198 torso CT scans (approximately 1.13 million axial slices) sourced from Massachusetts General Hospital (MGH) and the AutoPET challenge. Evaluation on 700 paired torso surface-CT cases demonstrated strong anatomical fidelity: organ volumes exhibited small mean percentage differences (range from -11.1% to 4.4%), and muscle/fat body composition metrics matched ground truth with strong correlation (range from 0.67 to 0.96). Lung localization had minimal bias (mean difference -2.5 mm), and surface completion significantly improved metrics (Chamfer distance: from 521.8 mm to 2.7 mm; Intersection-over-Union: from 0.87 to 0.98). Surf2CT establishes a new paradigm for non-invasive internal anatomical imaging using only external data, opening opportunities for home-based healthcare, preventive medicine, and personalized clinical assessments without the risks associated with conventional imaging techniques.
Cascaded 3D Diffusion Models for Whole-body 3D 18-F FDG PET/CT synthesis from Demographics
May 28, 2025Abstract:We propose a cascaded 3D diffusion model framework to synthesize high-fidelity 3D PET/CT volumes directly from demographic variables, addressing the growing need for realistic digital twins in oncologic imaging, virtual trials, and AI-driven data augmentation. Unlike deterministic phantoms, which rely on predefined anatomical and metabolic templates, our method employs a two-stage generative process. An initial score-based diffusion model synthesizes low-resolution PET/CT volumes from demographic variables alone, providing global anatomical structures and approximate metabolic activity. This is followed by a super-resolution residual diffusion model that refines spatial resolution. Our framework was trained on 18-F FDG PET/CT scans from the AutoPET dataset and evaluated using organ-wise volume and standardized uptake value (SUV) distributions, comparing synthetic and real data between demographic subgroups. The organ-wise comparison demonstrated strong concordance between synthetic and real images. In particular, most deviations in metabolic uptake values remained within 3-5% of the ground truth in subgroup analysis. These findings highlight the potential of cascaded 3D diffusion models to generate anatomically and metabolically accurate PET/CT images, offering a robust alternative to traditional phantoms and enabling scalable, population-informed synthetic imaging for clinical and research applications.
OWT: A Foundational Organ-Wise Tokenization Framework for Medical Imaging
May 08, 2025Abstract:Recent advances in representation learning often rely on holistic, black-box embeddings that entangle multiple semantic components, limiting interpretability and generalization. These issues are especially critical in medical imaging. To address these limitations, we propose an Organ-Wise Tokenization (OWT) framework with a Token Group-based Reconstruction (TGR) training paradigm. Unlike conventional approaches that produce holistic features, OWT explicitly disentangles an image into separable token groups, each corresponding to a distinct organ or semantic entity. Our design ensures each token group encapsulates organ-specific information, boosting interpretability, generalization, and efficiency while allowing fine-grained control in downstream tasks. Experiments on CT and MRI datasets demonstrate the effectiveness of OWT in not only achieving strong image reconstruction and segmentation performance, but also enabling novel semantic-level generation and retrieval applications that are out of reach for standard holistic embedding methods. These findings underscore the potential of OWT as a foundational framework for semantically disentangled representation learning, offering broad scalability and applicability to real-world medical imaging scenarios and beyond.
MAST-Pro: Dynamic Mixture-of-Experts for Adaptive Segmentation of Pan-Tumors with Knowledge-Driven Prompts
Mar 18, 2025Abstract:Accurate tumor segmentation is crucial for cancer diagnosis and treatment. While foundation models have advanced general-purpose segmentation, existing methods still struggle with: (1) limited incorporation of medical priors, (2) imbalance between generic and tumor-specific features, and (3) high computational costs for clinical adaptation. To address these challenges, we propose MAST-Pro (Mixture-of-experts for Adaptive Segmentation of pan-Tumors with knowledge-driven Prompts), a novel framework that integrates dynamic Mixture-of-Experts (D-MoE) and knowledge-driven prompts for pan-tumor segmentation. Specifically, text and anatomical prompts provide domain-specific priors, guiding tumor representation learning, while D-MoE dynamically selects experts to balance generic and tumor-specific feature learning, improving segmentation accuracy across diverse tumor types. To enhance efficiency, we employ Parameter-Efficient Fine-Tuning (PEFT), optimizing MAST-Pro with significantly reduced computational overhead. Experiments on multi-anatomical tumor datasets demonstrate that MAST-Pro outperforms state-of-the-art approaches, achieving up to a 5.20% improvement in average DSC while reducing trainable parameters by 91.04%, without compromising accuracy.
Prediction of Frozen Region Growth in Kidney Cryoablation Intervention Using a 3D Flow-Matching Model
Mar 06, 2025Abstract:This study presents a 3D flow-matching model designed to predict the progression of the frozen region (iceball) during kidney cryoablation. Precise intraoperative guidance is critical in cryoablation to ensure complete tumor eradication while preserving adjacent healthy tissue. However, conventional methods, typically based on physics driven or diffusion based simulations, are computationally demanding and often struggle to represent complex anatomical structures accurately. To address these limitations, our approach leverages intraoperative CT imaging to inform the model. The proposed 3D flow matching model is trained to learn a continuous deformation field that maps early-stage CT scans to future predictions. This transformation not only estimates the volumetric expansion of the iceball but also generates corresponding segmentation masks, effectively capturing spatial and morphological changes over time. Quantitative analysis highlights the model robustness, demonstrating strong agreement between predictions and ground-truth segmentations. The model achieves an Intersection over Union (IoU) score of 0.61 and a Dice coefficient of 0.75. By integrating real time CT imaging with advanced deep learning techniques, this approach has the potential to enhance intraoperative guidance in kidney cryoablation, improving procedural outcomes and advancing the field of minimally invasive surgery.
Developing a PET/CT Foundation Model for Cross-Modal Anatomical and Functional Imaging
Mar 04, 2025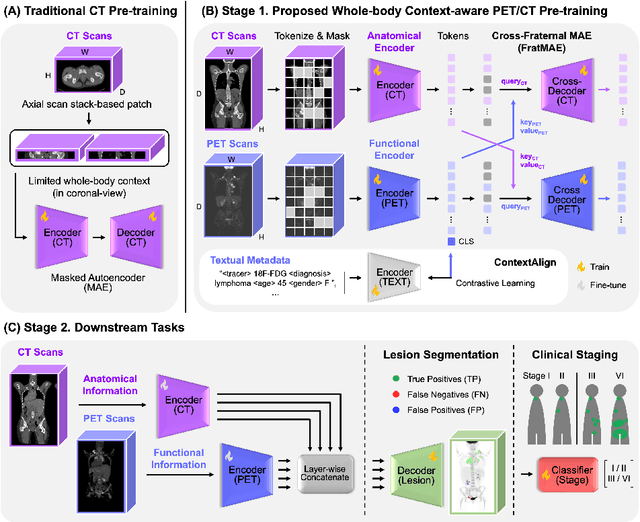
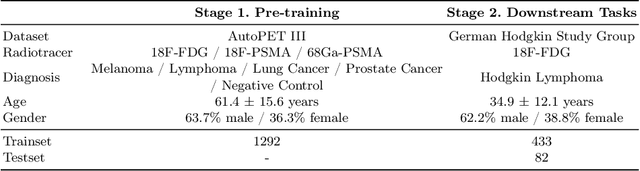

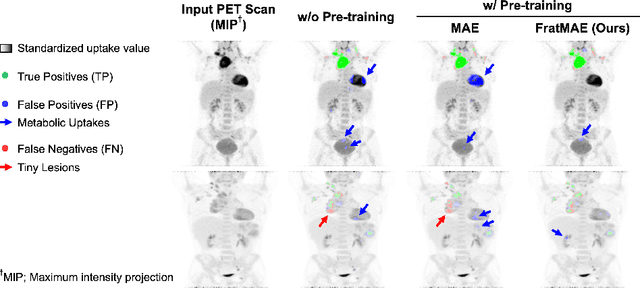
Abstract:In oncology, Positron Emission Tomography-Computed Tomography (PET/CT) is widely used in cancer diagnosis, staging, and treatment monitoring, as it combines anatomical details from CT with functional metabolic activity and molecular marker expression information from PET. However, existing artificial intelligence-driven PET/CT analyses rely predominantly on task-specific models trained from scratch or on limited datasets, limiting their generalizability and robustness. To address this, we propose a foundation model approach specifically designed for multimodal PET/CT imaging. We introduce the Cross-Fraternal Twin Masked Autoencoder (FratMAE), a novel framework that effectively integrates whole-body anatomical and functional or molecular information. FratMAE employs separate Vision Transformer (ViT) encoders for PET and CT scans, along with cross-attention decoders that enable synergistic interactions between modalities during masked autoencoder training. Additionally, it incorporates textual metadata to enhance PET representation learning. By pre-training on PET/CT datasets, FratMAE captures intricate cross-modal relationships and global uptake patterns, achieving superior performance on downstream tasks and demonstrating its potential as a generalizable foundation model.
Improving Factuality of 3D Brain MRI Report Generation with Paired Image-domain Retrieval and Text-domain Augmentation
Nov 23, 2024



Abstract:Acute ischemic stroke (AIS) requires time-critical management, with hours of delayed intervention leading to an irreversible disability of the patient. Since diffusion weighted imaging (DWI) using the magnetic resonance image (MRI) plays a crucial role in the detection of AIS, automated prediction of AIS from DWI has been a research topic of clinical importance. While text radiology reports contain the most relevant clinical information from the image findings, the difficulty of mapping across different modalities has limited the factuality of conventional direct DWI-to-report generation methods. Here, we propose paired image-domain retrieval and text-domain augmentation (PIRTA), a cross-modal retrieval-augmented generation (RAG) framework for providing clinician-interpretative AIS radiology reports with improved factuality. PIRTA mitigates the need for learning cross-modal mapping, which poses difficulty in image-to-text generation, by casting the cross-modal mapping problem as an in-domain retrieval of similar DWI images that have paired ground-truth text radiology reports. By exploiting the retrieved radiology reports to augment the report generation process of the query image, we show by experiments with extensive in-house and public datasets that PIRTA can accurately retrieve relevant reports from 3D DWI images. This approach enables the generation of radiology reports with significantly higher accuracy compared to direct image-to-text generation using state-of-the-art multimodal language models.
OTSeg: Multi-prompt Sinkhorn Attention for Zero-Shot Semantic Segmentation
Mar 21, 2024



Abstract:The recent success of CLIP has demonstrated promising results in zero-shot semantic segmentation by transferring muiltimodal knowledge to pixel-level classification. However, leveraging pre-trained CLIP knowledge to closely align text embeddings with pixel embeddings still has limitations in existing approaches. To address this issue, we propose OTSeg, a novel multimodal attention mechanism aimed at enhancing the potential of multiple text prompts for matching associated pixel embeddings. We first propose Multi-Prompts Sinkhorn (MPS) based on the Optimal Transport (OT) algorithm, which leads multiple text prompts to selectively focus on various semantic features within image pixels. Moreover, inspired by the success of Sinkformers in unimodal settings, we introduce the extension of MPS, called Multi-Prompts Sinkhorn Attention (MPSA), which effectively replaces cross-attention mechanisms within Transformer framework in multimodal settings. Through extensive experiments, we demonstrate that OTSeg achieves state-of-the-art (SOTA) performance with significant gains on Zero-Shot Semantic Segmentation (ZS3) tasks across three benchmark datasets.
RO-LLaMA: Generalist LLM for Radiation Oncology via Noise Augmentation and Consistency Regularization
Nov 27, 2023Abstract:Recent advancements in Artificial Intelligence (AI) have profoundly influenced medical fields, by providing tools to reduce clinical workloads. However, most AI models are constrained to execute uni-modal tasks, in stark contrast to the comprehensive approaches utilized by medical professionals. To address this, here we present RO-LLaMA, a versatile generalist large language model (LLM) tailored for the field of radiation oncology. This model seamlessly covers a wide range of the workflow of radiation oncologists, adept at various tasks such as clinical report summarization, radiation therapy plan suggestion, and plan-guided therapy target volume segmentation. In particular, to maximize the end-to-end performance, we further present a novel Consistency Embedding Fine-Tuning (CEFTune) technique, which boosts LLM's robustness to additional errors at the intermediates while preserving the capability of handling clean inputs, and creatively transform this concept into LLM-driven segmentation framework as Consistency Embedding Segmentation (CESEG). Experimental results on multi-centre cohort sets demonstrate our proposed RO-LLaMA's promising performance for diverse tasks with generalization capabilities.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge