Yueping Liu
Department of Pathology, The Fourth Hospital of Hebei Medical University, Hebei, China
PathBench: A comprehensive comparison benchmark for pathology foundation models towards precision oncology
May 26, 2025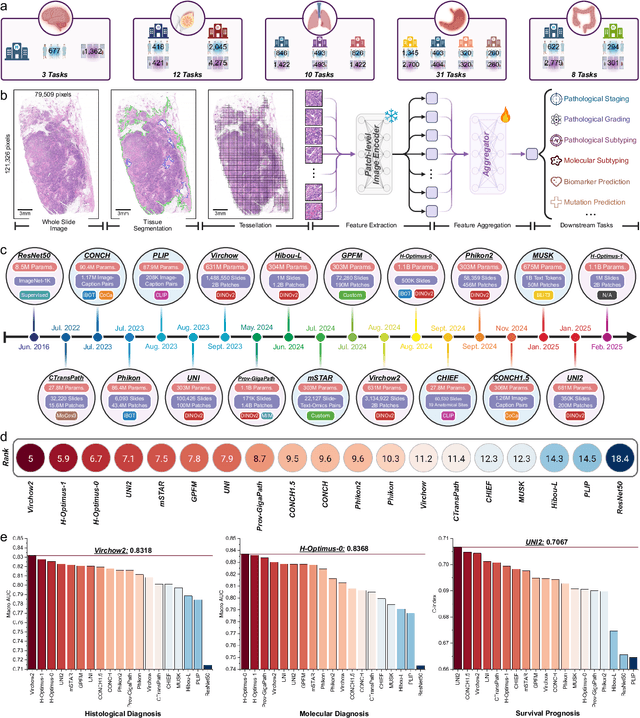



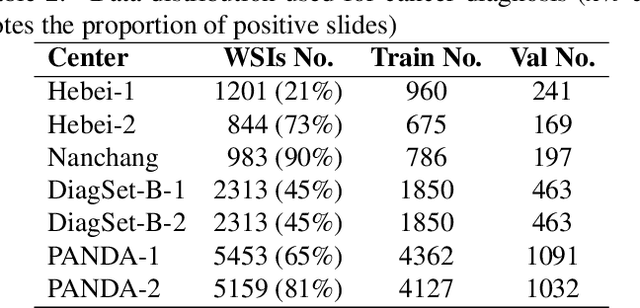
Abstract:The emergence of pathology foundation models has revolutionized computational histopathology, enabling highly accurate, generalized whole-slide image analysis for improved cancer diagnosis, and prognosis assessment. While these models show remarkable potential across cancer diagnostics and prognostics, their clinical translation faces critical challenges including variability in optimal model across cancer types, potential data leakage in evaluation, and lack of standardized benchmarks. Without rigorous, unbiased evaluation, even the most advanced PFMs risk remaining confined to research settings, delaying their life-saving applications. Existing benchmarking efforts remain limited by narrow cancer-type focus, potential pretraining data overlaps, or incomplete task coverage. We present PathBench, the first comprehensive benchmark addressing these gaps through: multi-center in-hourse datasets spanning common cancers with rigorous leakage prevention, evaluation across the full clinical spectrum from diagnosis to prognosis, and an automated leaderboard system for continuous model assessment. Our framework incorporates large-scale data, enabling objective comparison of PFMs while reflecting real-world clinical complexity. All evaluation data comes from private medical providers, with strict exclusion of any pretraining usage to avoid data leakage risks. We have collected 15,888 WSIs from 8,549 patients across 10 hospitals, encompassing over 64 diagnosis and prognosis tasks. Currently, our evaluation of 19 PFMs shows that Virchow2 and H-Optimus-1 are the most effective models overall. This work provides researchers with a robust platform for model development and offers clinicians actionable insights into PFM performance across diverse clinical scenarios, ultimately accelerating the translation of these transformative technologies into routine pathology practice.
Federated contrastive learning models for prostate cancer diagnosis and Gleason grading
Feb 17, 2023
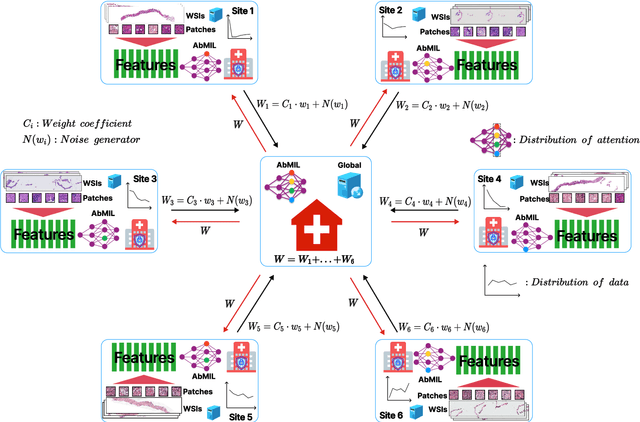
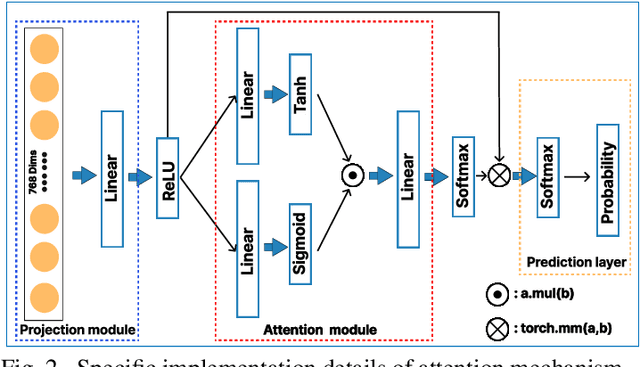
Abstract:The application effect of artificial intelligence (AI) in the field of medical imaging is remarkable. Robust AI model training requires large datasets, but data collection faces communication, ethics, and privacy protection constraints. Fortunately, federated learning can solve the above problems by coordinating multiple clients to train the model without sharing the original data. In this study, we design a federated contrastive learning framework (FCL) for large-scale pathology images and the heterogeneity challenges. It enhances the model's generalization ability by maximizing the attention consistency between the local client and server models. To alleviate the privacy leakage problem when transferring parameters and verify the robustness of FCL, we use differential privacy to further protect the model by adding noise. We evaluate the effectiveness of FCL on the cancer diagnosis task and Gleason grading task on 19,635 prostate cancer WSIs from multiple clients. In the diagnosis task, the average AUC of 7 clients is 95% when the categories are relatively balanced, and our FCL achieves 97%. In the Gleason grading task, the average Kappa of 6 clients is 0.74, and the Kappa of FCL reaches 0.84. Furthermore, we also validate the robustness of the model on external datasets(one public dataset and two private datasets). In addition, to better explain the classification effect of the model, we show whether the model focuses on the lesion area by drawing a heatmap. Finally, FCL brings a robust, accurate, low-cost AI training model to biomedical research, effectively protecting medical data privacy.
Blind deblurring for microscopic pathology images using deep learning networks
Nov 24, 2020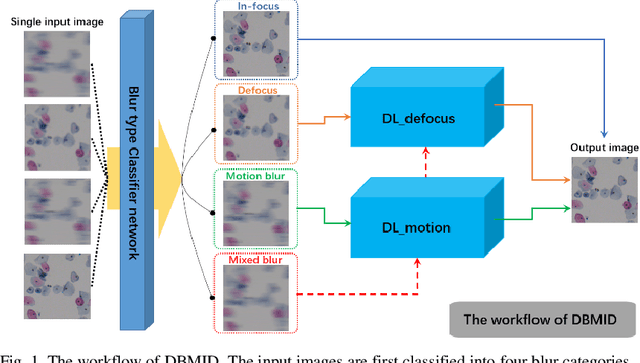
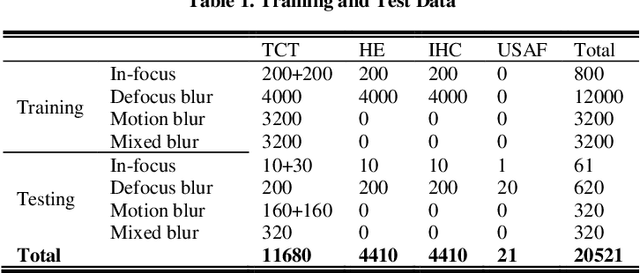
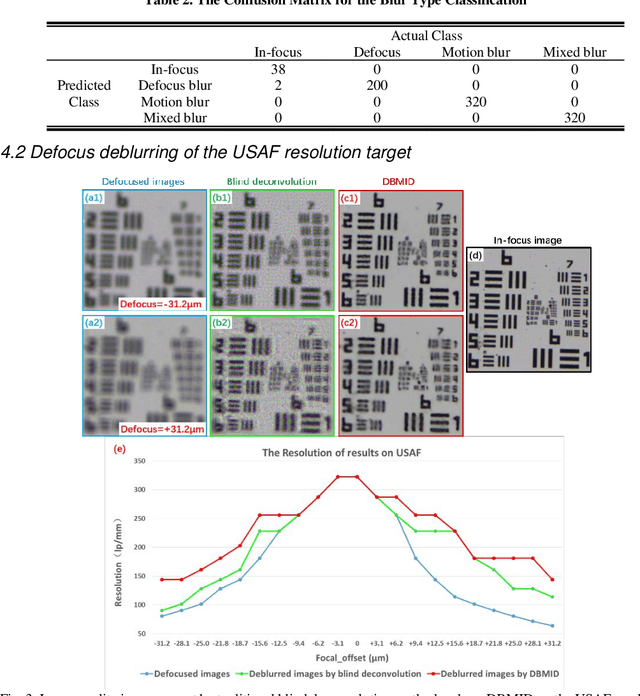
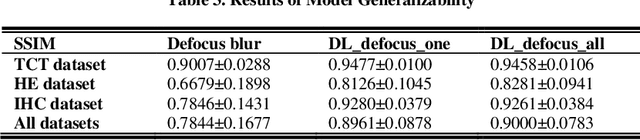
Abstract:Artificial Intelligence (AI)-powered pathology is a revolutionary step in the world of digital pathology and shows great promise to increase both diagnosis accuracy and efficiency. However, defocus and motion blur can obscure tissue or cell characteristics hence compromising AI algorithms'accuracy and robustness in analyzing the images. In this paper, we demonstrate a deep-learning-based approach that can alleviate the defocus and motion blur of a microscopic image and output a sharper and cleaner image with retrieved fine details without prior knowledge of the blur type, blur extent and pathological stain. In this approach, a deep learning classifier is first trained to identify the image blur type. Then, two encoder-decoder networks are trained and used alone or in combination to deblur the input image. It is an end-to-end approach and introduces no corrugated artifacts as traditional blind deconvolution methods do. We test our approach on different types of pathology specimens and demonstrate great performance on image blur correction and the subsequent improvement on the diagnosis outcome of AI algorithms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge