Xiaoming Qi
Dynamic Allocation Hypernetwork with Adaptive Model Recalibration for Federated Continual Learning
Mar 25, 2025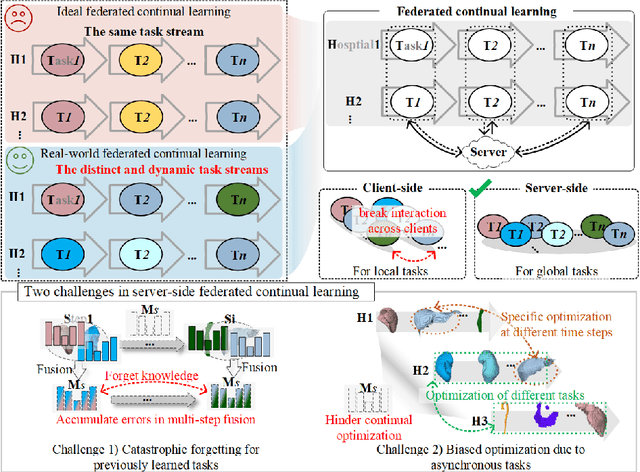
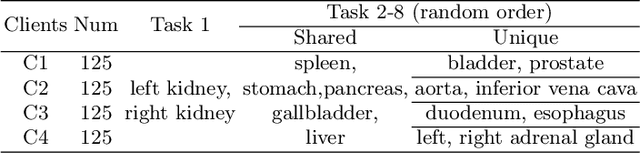
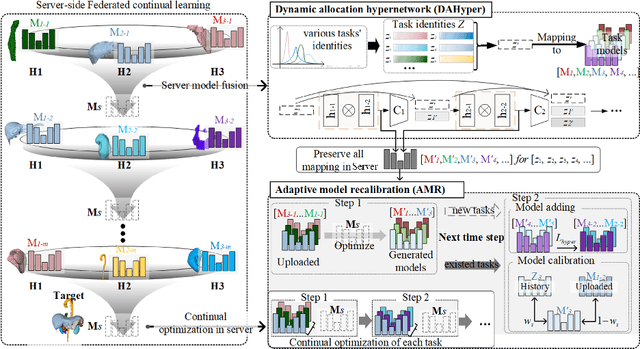
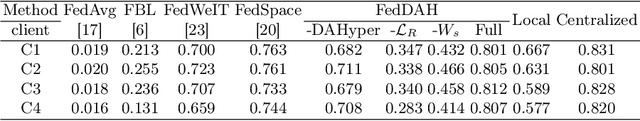
Abstract:Federated continual learning (FCL) offers an emerging pattern to facilitate the applicability of federated learning (FL) in real-world scenarios, where tasks evolve dynamically and asynchronously across clients, especially in medical scenario. Existing server-side FCL methods in nature domain construct a continually learnable server model by client aggregation on all-involved tasks. However, they are challenged by: (1) Catastrophic forgetting for previously learned tasks, leading to error accumulation in server model, making it difficult to sustain comprehensive knowledge across all tasks. (2) Biased optimization due to asynchronous tasks handled across different clients, leading to the collision of optimization targets of different clients at the same time steps. In this work, we take the first step to propose a novel server-side FCL pattern in medical domain, Dynamic Allocation Hypernetwork with adaptive model recalibration (FedDAH). It is to facilitate collaborative learning under the distinct and dynamic task streams across clients. To alleviate the catastrophic forgetting, we propose a dynamic allocation hypernetwork (DAHyper) where a continually updated hypernetwork is designed to manage the mapping between task identities and their associated model parameters, enabling the dynamic allocation of the model across clients. For the biased optimization, we introduce a novel adaptive model recalibration (AMR) to incorporate the candidate changes of historical models into current server updates, and assign weights to identical tasks across different time steps based on the similarity for continual optimization. Extensive experiments on the AMOS dataset demonstrate the superiority of our FedDAH to other FCL methods on sites with different task streams. The code is available:https://github.com/jinlab-imvr/FedDAH.
DSCENet: Dynamic Screening and Clinical-Enhanced Multimodal Fusion for MPNs Subtype Classification
Jul 11, 2024



Abstract:The precise subtype classification of myeloproliferative neoplasms (MPNs) based on multimodal information, which assists clinicians in diagnosis and long-term treatment plans, is of great clinical significance. However, it remains a great challenging task due to the lack of diagnostic representativeness for local patches and the absence of diagnostic-relevant features from a single modality. In this paper, we propose a Dynamic Screening and Clinical-Enhanced Network (DSCENet) for the subtype classification of MPNs on the multimodal fusion of whole slide images (WSIs) and clinical information. (1) A dynamic screening module is proposed to flexibly adapt the feature learning of local patches, reducing the interference of irrelevant features and enhancing their diagnostic representativeness. (2) A clinical-enhanced fusion module is proposed to integrate clinical indicators to explore complementary features across modalities, providing comprehensive diagnostic information. Our approach has been validated on the real clinical data, achieving an increase of 7.91% AUC and 16.89% accuracy compared with the previous state-of-the-art (SOTA) methods. The code is available at https://github.com/yuanzhang7/DSCENet.
FedSODA: Federated Cross-assessment and Dynamic Aggregation for Histopathology Segmentation
Dec 20, 2023


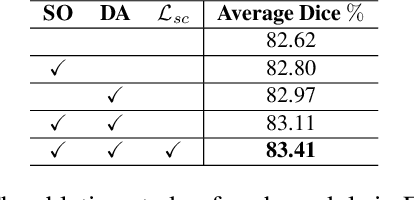
Abstract:Federated learning (FL) for histopathology image segmentation involving multiple medical sites plays a crucial role in advancing the field of accurate disease diagnosis and treatment. However, it is still a task of great challenges due to the sample imbalance across clients and large data heterogeneity from disparate organs, variable segmentation tasks, and diverse distribution. Thus, we propose a novel FL approach for histopathology nuclei and tissue segmentation, FedSODA, via synthetic-driven cross-assessment operation (SO) and dynamic stratified-layer aggregation (DA). Our SO constructs a cross-assessment strategy to connect clients and mitigate the representation bias under sample imbalance. Our DA utilizes layer-wise interaction and dynamic aggregation to diminish heterogeneity and enhance generalization. The effectiveness of our FedSODA has been evaluated on the most extensive histopathology image segmentation dataset from 7 independent datasets. The code is available at https://github.com/yuanzhang7/FedSODA.
Dynamic Snake Convolution based on Topological Geometric Constraints for Tubular Structure Segmentation
Jul 17, 2023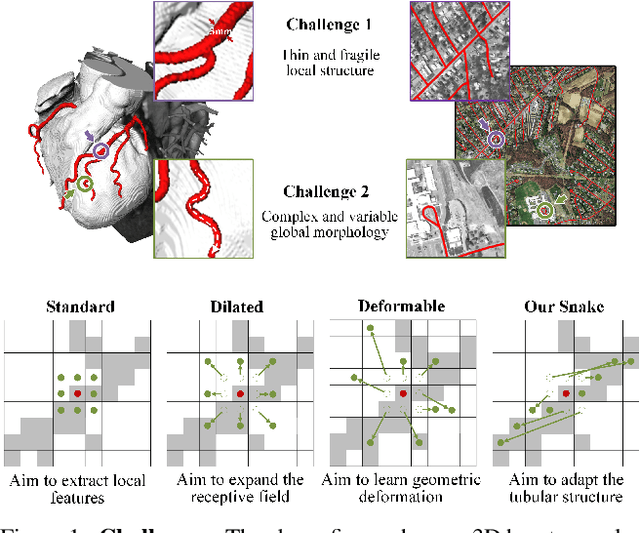

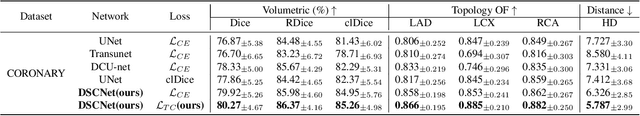
Abstract:Accurate segmentation of topological tubular structures, such as blood vessels and roads, is crucial in various fields, ensuring accuracy and efficiency in downstream tasks. However, many factors complicate the task, including thin local structures and variable global morphologies. In this work, we note the specificity of tubular structures and use this knowledge to guide our DSCNet to simultaneously enhance perception in three stages: feature extraction, feature fusion, and loss constraint. First, we propose a dynamic snake convolution to accurately capture the features of tubular structures by adaptively focusing on slender and tortuous local structures. Subsequently, we propose a multi-view feature fusion strategy to complement the attention to features from multiple perspectives during feature fusion, ensuring the retention of important information from different global morphologies. Finally, a continuity constraint loss function, based on persistent homology, is proposed to constrain the topological continuity of the segmentation better. Experiments on 2D and 3D datasets show that our DSCNet provides better accuracy and continuity on the tubular structure segmentation task compared with several methods. Our codes will be publicly available.
MNet: Rethinking 2D/3D Networks for Anisotropic Medical Image Segmentation
May 10, 2022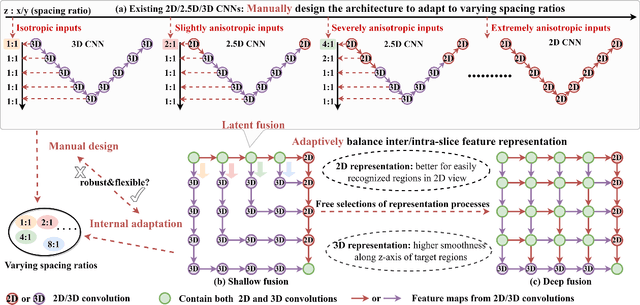
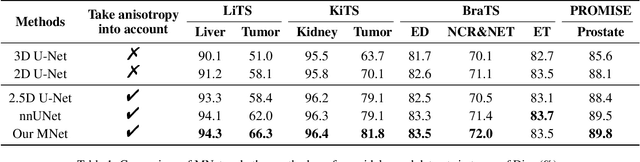
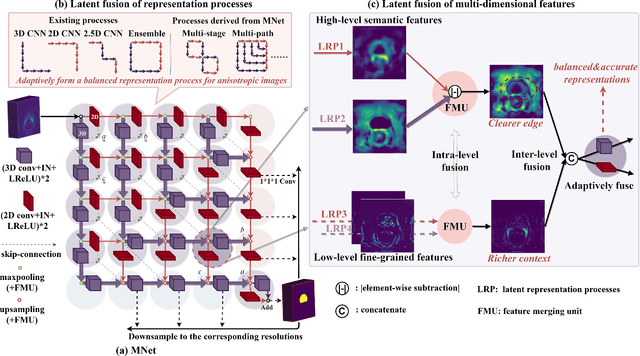

Abstract:The nature of thick-slice scanning causes severe inter-slice discontinuities of 3D medical images, and the vanilla 2D/3D convolutional neural networks (CNNs) fail to represent sparse inter-slice information and dense intra-slice information in a balanced way, leading to severe underfitting to inter-slice features (for vanilla 2D CNNs) and overfitting to noise from long-range slices (for vanilla 3D CNNs). In this work, a novel mesh network (MNet) is proposed to balance the spatial representation inter axes via learning. 1) Our MNet latently fuses plenty of representation processes by embedding multi-dimensional convolutions deeply into basic modules, making the selections of representation processes flexible, thus balancing representation for sparse inter-slice information and dense intra-slice information adaptively. 2) Our MNet latently fuses multi-dimensional features inside each basic module, simultaneously taking the advantages of 2D (high segmentation accuracy of the easily recognized regions in 2D view) and 3D (high smoothness of 3D organ contour) representations, thus obtaining more accurate modeling for target regions. Comprehensive experiments are performed on four public datasets (CT\&MR), the results consistently demonstrate the proposed MNet outperforms the other methods. The code and datasets are available at: https://github.com/zfdong-code/MNet
EnMcGAN: Adversarial Ensemble Learning for 3D Complete Renal Structures Segmentation
Jun 08, 2021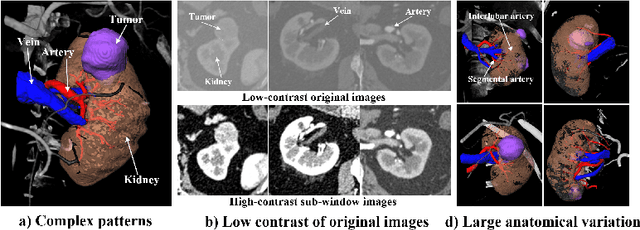
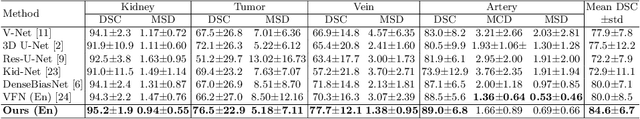
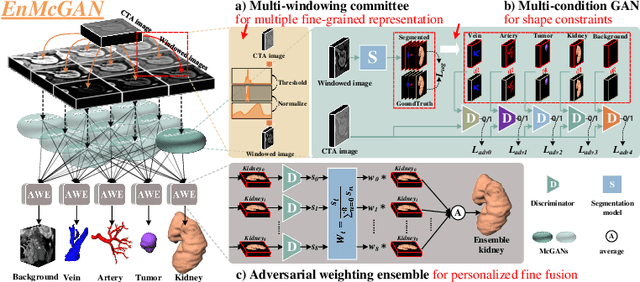
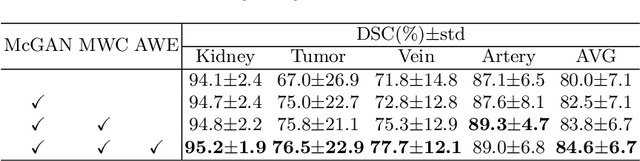
Abstract:3D complete renal structures(CRS) segmentation targets on segmenting the kidneys, tumors, renal arteries and veins in one inference. Once successful, it will provide preoperative plans and intraoperative guidance for laparoscopic partial nephrectomy(LPN), playing a key role in the renal cancer treatment. However, no success has been reported in 3D CRS segmentation due to the complex shapes of renal structures, low contrast and large anatomical variation. In this study, we utilize the adversarial ensemble learning and propose Ensemble Multi-condition GAN(EnMcGAN) for 3D CRS segmentation for the first time. Its contribution is three-fold. 1)Inspired by windowing, we propose the multi-windowing committee which divides CTA image into multiple narrow windows with different window centers and widths enhancing the contrast for salient boundaries and soft tissues. And then, it builds an ensemble segmentation model on these narrow windows to fuse the segmentation superiorities and improve whole segmentation quality. 2)We propose the multi-condition GAN which equips the segmentation model with multiple discriminators to encourage the segmented structures meeting their real shape conditions, thus improving the shape feature extraction ability. 3)We propose the adversarial weighted ensemble module which uses the trained discriminators to evaluate the quality of segmented structures, and normalizes these evaluation scores for the ensemble weights directed at the input image, thus enhancing the ensemble results. 122 patients are enrolled in this study and the mean Dice coefficient of the renal structures achieves 84.6%. Extensive experiments with promising results on renal structures reveal powerful segmentation accuracy and great clinical significance in renal cancer treatment.
Deep Reinforcement Learning for Imbalanced Classification
Jan 05, 2019



Abstract:Data in real-world application often exhibit skewed class distribution which poses an intense challenge for machine learning. Conventional classification algorithms are not effective in the case of imbalanced data distribution, and may fail when the data distribution is highly imbalanced. To address this issue, we propose a general imbalanced classification model based on deep reinforcement learning. We formulate the classification problem as a sequential decision-making process and solve it by deep Q-learning network. The agent performs a classification action on one sample at each time step, and the environment evaluates the classification action and returns a reward to the agent. The reward from minority class sample is larger so the agent is more sensitive to the minority class. The agent finally finds an optimal classification policy in imbalanced data under the guidance of specific reward function and beneficial learning environment. Experiments show that our proposed model outperforms the other imbalanced classification algorithms, and it can identify more minority samples and has great classification performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge