Xiangyu Meng
Identity-GRPO: Optimizing Multi-Human Identity-preserving Video Generation via Reinforcement Learning
Oct 16, 2025Abstract:While advanced methods like VACE and Phantom have advanced video generation for specific subjects in diverse scenarios, they struggle with multi-human identity preservation in dynamic interactions, where consistent identities across multiple characters are critical. To address this, we propose Identity-GRPO, a human feedback-driven optimization pipeline for refining multi-human identity-preserving video generation. First, we construct a video reward model trained on a large-scale preference dataset containing human-annotated and synthetic distortion data, with pairwise annotations focused on maintaining human consistency throughout the video. We then employ a GRPO variant tailored for multi-human consistency, which greatly enhances both VACE and Phantom. Through extensive ablation studies, we evaluate the impact of annotation quality and design choices on policy optimization. Experiments show that Identity-GRPO achieves up to 18.9% improvement in human consistency metrics over baseline methods, offering actionable insights for aligning reinforcement learning with personalized video generation.
Gene-MOE: A Sparsely-gated Framework for Pan-Cancer Genomic Analysis
Nov 29, 2023Abstract:Analyzing the genomic information from the Pan-Cancer database can help us understand cancer-related factors and contribute to the cancer diagnosis and prognosis. However, existing computational methods and deep learning methods can not effectively find the deep correlations between tens of thousands of genes, which leads to precision loss. In this paper, we proposed a novel pretrained model called Gene-MOE to learn the general feature representations of the Pan-Cancer dataset and transfer the pretrained weights to the downstream tasks. The Gene-MOE fully exploits the mixture of expert (MOE) layers to learn rich feature representations of high-dimensional genes. At the same time, we build a mixture of attention expert (MOAE) model to learn the deep semantic relationships within genetic features. Finally, we proposed a new self-supervised pretraining strategy including loss function design, data enhancement, and optimization strategy to train the Gene-MOE and further improve the performance for the downstream analysis. We carried out cancer classification and survival analysis experiments based on the Gene-MOE. According to the survival analysis results on 14 cancer types, using Gene-MOE outperformed state-of-the-art models on 12 cancer types. According to the classification results, the total accuracy of the classification model for 33 cancer classifications reached 95.2\%. Through detailed feature analysis, we found the Gene-MOE model can learn rich feature representations of high-dimensional genes.
ASF-Net: Robust Video Deraining via Temporal Alignment and Online Adaptive Learning
Sep 02, 2023



Abstract:In recent times, learning-based methods for video deraining have demonstrated commendable results. However, there are two critical challenges that these methods are yet to address: exploiting temporal correlations among adjacent frames and ensuring adaptability to unknown real-world scenarios. To overcome these challenges, we explore video deraining from a paradigm design perspective to learning strategy construction. Specifically, we propose a new computational paradigm, Alignment-Shift-Fusion Network (ASF-Net), which incorporates a temporal shift module. This module is novel to this field and provides deeper exploration of temporal information by facilitating the exchange of channel-level information within the feature space. To fully discharge the model's characterization capability, we further construct a LArge-scale RAiny video dataset (LARA) which also supports the development of this community. On the basis of the newly-constructed dataset, we explore the parameters learning process by developing an innovative re-degraded learning strategy. This strategy bridges the gap between synthetic and real-world scenes, resulting in stronger scene adaptability. Our proposed approach exhibits superior performance in three benchmarks and compelling visual quality in real-world scenarios, underscoring its efficacy. The code is available at https://github.com/vis-opt-group/ASF-Net.
High-risk Factor Prediction in Lung Cancer Using Thin CT Scans: An Attention-Enhanced Graph Convolutional Network Approach
Aug 27, 2023



Abstract:Lung cancer, particularly in its advanced stages, remains a leading cause of death globally. Though early detection via low-dose computed tomography (CT) is promising, the identification of high-risk factors crucial for surgical mode selection remains a challenge. Addressing this, our study introduces an Attention-Enhanced Graph Convolutional Network (AE-GCN) model to classify whether there are high-risk factors in stage I lung cancer based on the preoperative CT images. This will aid surgeons in determining the optimal surgical method before the operation. Unlike previous studies that relied on 3D patch techniques to represent nodule spatial features, our method employs a GCN model to capture the spatial characteristics of pulmonary nodules. Specifically, we regard each slice of the nodule as a graph vertex, and the inherent spatial relationships between slices form the edges. Then, to enhance the expression of nodule features, we integrated both channel and spatial attention mechanisms with a pre-trained VGG model for adaptive feature extraction from pulmonary nodules. Lastly, the effectiveness of the proposed method is demonstrated using real-world data collected from the hospitals, thereby emphasizing its potential utility in the clinical practice.
Learning nonlinear dynamics in synchronization of knowledge-based leader-following networks
Dec 29, 2021



Abstract:Knowledge-based leader-following synchronization problem of heterogeneous nonlinear multi-agent systems is challenging since the leader's dynamic information is unknown to all follower nodes. This paper proposes a learning-based fully distributed observer for a class of nonlinear leader systems, which can simultaneously learn the leader's dynamics and states. The class of leader dynamics considered here does not require a bounded Jacobian matrix. Based on this learning-based distributed observer, we further synthesize an adaptive distributed control law for solving the leader-following synchronization problem of multiple Euler-Lagrange systems subject to an uncertain nonlinear leader system. The results are illustrated by a simulation example.
Exploiting full Resolution Feature Context for Liver Tumor and Vessel Segmentation via Fusion Encoder: Application to Liver Tumor and Vessel 3D reconstruction
Nov 26, 2021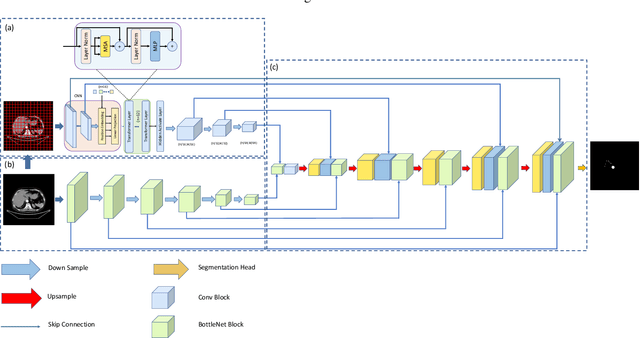
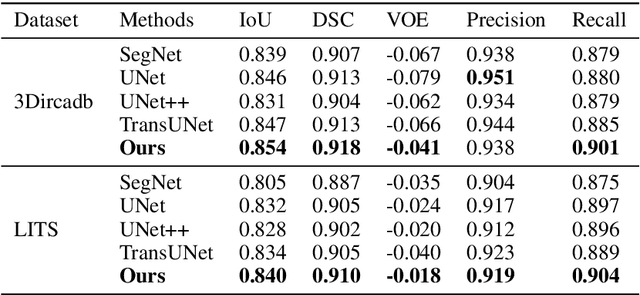
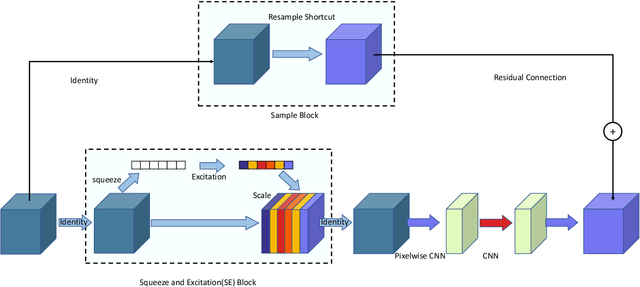
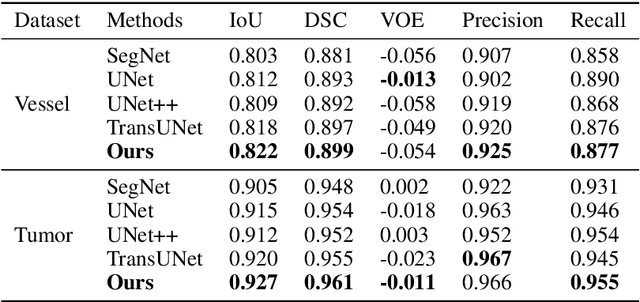
Abstract:Liver cancer is one of the most common malignant diseases in the world. Segmentation and labeling of liver tumors and blood vessels in CT images can provide convenience for doctors in liver tumor diagnosis and surgical intervention. In the past decades, automatic CT segmentation methods based on deep learning have received widespread attention in the medical field. Many state-of-the-art segmentation algorithms appeared during this period. Yet, most of the existing segmentation methods only care about the local feature context and have a perception defect in the global relevance of medical images, which significantly affects the segmentation effect of liver tumors and blood vessels. We introduce a multi-scale feature context fusion network called TransFusionNet based on Transformer and SEBottleNet. This network can accurately detect and identify the details of the region of interest of the liver vessel, meanwhile it can improve the recognition of morphologic margins of liver tumors by exploiting the global information of CT images. Experiments show that TransFusionNet is better than the state-of-the-art method on both the public dataset LITS and 3Dircadb and our clinical dataset. Finally, we propose an automatic 3D reconstruction algorithm based on the trained model. The algorithm can complete the reconstruction quickly and accurately in 1 second.
Comparison of Centralized and Decentralized Approaches in Cooperative Coverage Problems with Energy-Constrained Agents
Aug 26, 2020



Abstract:A multi-agent coverage problem is considered with energy-constrained agents. The objective of this paper is to compare the coverage performance between centralized and decentralized approaches. To this end, a near-optimal centralized coverage control method is developed under energy depletion and repletion constraints. The optimal coverage formation corresponds to the locations of agents where the coverage performance is maximized. The optimal charging formation corresponds to the locations of agents with one agent fixed at the charging station and the remaining agents maximizing the coverage performance. We control the behavior of this cooperative multi-agent system by switching between the optimal coverage formation and the optimal charging formation. Finally, the optimal dwell times at coverage locations, charging time, and agent trajectories are determined so as to maximize coverage over a given time interval. In particular, our controller guarantees that at any time there is at most one agent leaving the team for energy repletion.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge