Huanhuan Dai
Gene-MOE: A Sparsely-gated Framework for Pan-Cancer Genomic Analysis
Nov 29, 2023Abstract:Analyzing the genomic information from the Pan-Cancer database can help us understand cancer-related factors and contribute to the cancer diagnosis and prognosis. However, existing computational methods and deep learning methods can not effectively find the deep correlations between tens of thousands of genes, which leads to precision loss. In this paper, we proposed a novel pretrained model called Gene-MOE to learn the general feature representations of the Pan-Cancer dataset and transfer the pretrained weights to the downstream tasks. The Gene-MOE fully exploits the mixture of expert (MOE) layers to learn rich feature representations of high-dimensional genes. At the same time, we build a mixture of attention expert (MOAE) model to learn the deep semantic relationships within genetic features. Finally, we proposed a new self-supervised pretraining strategy including loss function design, data enhancement, and optimization strategy to train the Gene-MOE and further improve the performance for the downstream analysis. We carried out cancer classification and survival analysis experiments based on the Gene-MOE. According to the survival analysis results on 14 cancer types, using Gene-MOE outperformed state-of-the-art models on 12 cancer types. According to the classification results, the total accuracy of the classification model for 33 cancer classifications reached 95.2\%. Through detailed feature analysis, we found the Gene-MOE model can learn rich feature representations of high-dimensional genes.
Exploiting full Resolution Feature Context for Liver Tumor and Vessel Segmentation via Fusion Encoder: Application to Liver Tumor and Vessel 3D reconstruction
Nov 26, 2021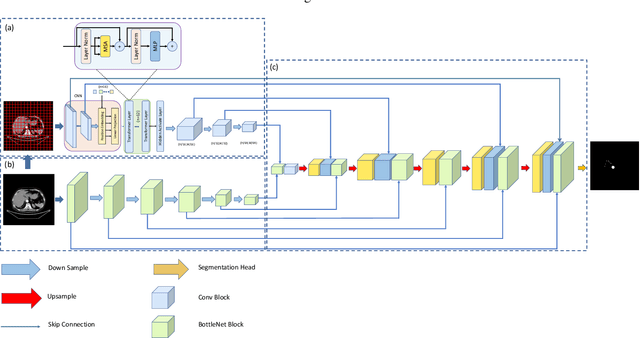
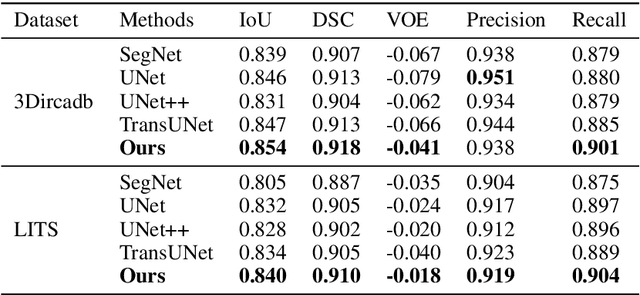
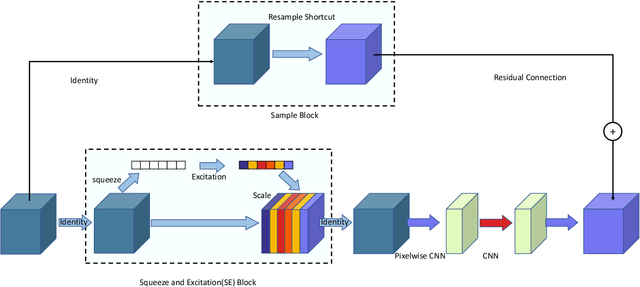
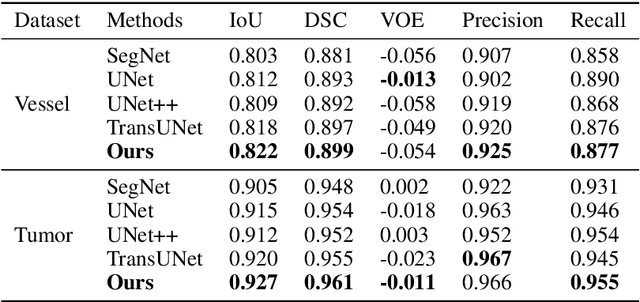
Abstract:Liver cancer is one of the most common malignant diseases in the world. Segmentation and labeling of liver tumors and blood vessels in CT images can provide convenience for doctors in liver tumor diagnosis and surgical intervention. In the past decades, automatic CT segmentation methods based on deep learning have received widespread attention in the medical field. Many state-of-the-art segmentation algorithms appeared during this period. Yet, most of the existing segmentation methods only care about the local feature context and have a perception defect in the global relevance of medical images, which significantly affects the segmentation effect of liver tumors and blood vessels. We introduce a multi-scale feature context fusion network called TransFusionNet based on Transformer and SEBottleNet. This network can accurately detect and identify the details of the region of interest of the liver vessel, meanwhile it can improve the recognition of morphologic margins of liver tumors by exploiting the global information of CT images. Experiments show that TransFusionNet is better than the state-of-the-art method on both the public dataset LITS and 3Dircadb and our clinical dataset. Finally, we propose an automatic 3D reconstruction algorithm based on the trained model. The algorithm can complete the reconstruction quickly and accurately in 1 second.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge