Silvia Ingala
for the ALFA study
MERA: Multimodal and Multiscale Self-Explanatory Model with Considerably Reduced Annotation for Lung Nodule Diagnosis
Apr 27, 2025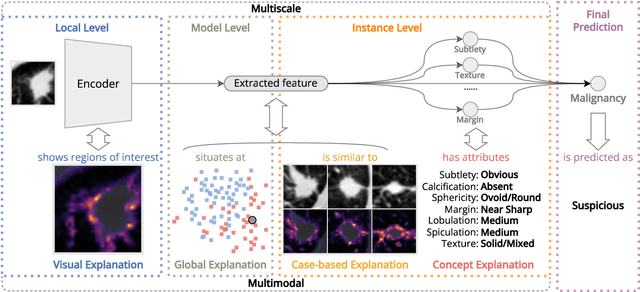
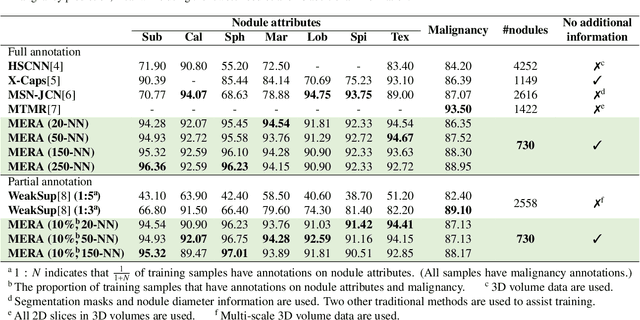
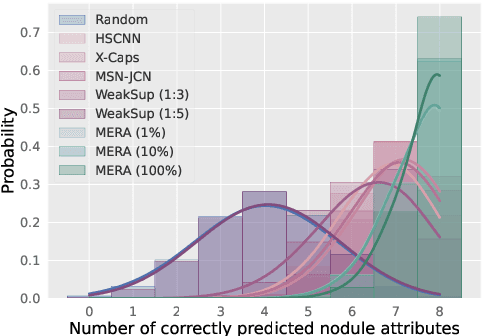
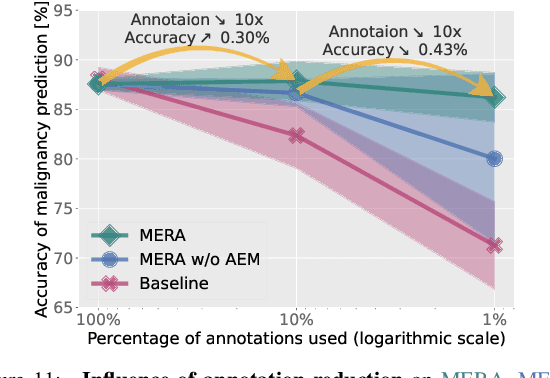
Abstract:Lung cancer, a leading cause of cancer-related deaths globally, emphasises the importance of early detection for better patient outcomes. Pulmonary nodules, often early indicators of lung cancer, necessitate accurate, timely diagnosis. Despite Explainable Artificial Intelligence (XAI) advances, many existing systems struggle providing clear, comprehensive explanations, especially with limited labelled data. This study introduces MERA, a Multimodal and Multiscale self-Explanatory model designed for lung nodule diagnosis with considerably Reduced Annotation requirements. MERA integrates unsupervised and weakly supervised learning strategies (self-supervised learning techniques and Vision Transformer architecture for unsupervised feature extraction) and a hierarchical prediction mechanism leveraging sparse annotations via semi-supervised active learning in the learned latent space. MERA explains its decisions on multiple levels: model-level global explanations via semantic latent space clustering, instance-level case-based explanations showing similar instances, local visual explanations via attention maps, and concept explanations using critical nodule attributes. Evaluations on the public LIDC dataset show MERA's superior diagnostic accuracy and self-explainability. With only 1% annotated samples, MERA achieves diagnostic accuracy comparable to or exceeding state-of-the-art methods requiring full annotation. The model's inherent design delivers comprehensive, robust, multilevel explanations aligned closely with clinical practice, enhancing trustworthiness and transparency. Demonstrated viability of unsupervised and weakly supervised learning lowers the barrier to deploying diagnostic AI in broader medical domains. Our complete code is open-source available: https://github.com/diku-dk/credanno.
Local Gamma Augmentation for Ischemic Stroke Lesion Segmentation on MRI
Jan 12, 2024Abstract:The identification and localisation of pathological tissues in medical images continues to command much attention among deep learning practitioners. When trained on abundant datasets, deep neural networks can match or exceed human performance. However, the scarcity of annotated data complicates the training of these models. Data augmentation techniques can compensate for a lack of training samples. However, many commonly used augmentation methods can fail to provide meaningful samples during model fitting. We present local gamma augmentation, a technique for introducing new instances of intensities in pathological tissues. We leverage local gamma augmentation to compensate for a bias in intensities corresponding to ischemic stroke lesions in human brain MRIs. On three datasets, we show how local gamma augmentation can improve the image-level sensitivity of a deep neural network tasked with ischemic lesion segmentation on magnetic resonance images.
Where is VALDO? VAscular Lesions Detection and segmentatiOn challenge at MICCAI 2021
Aug 15, 2022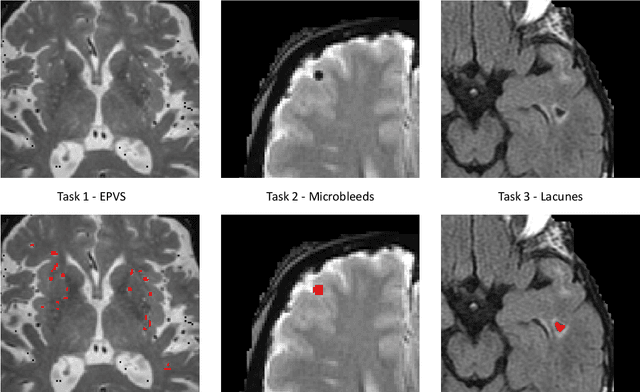
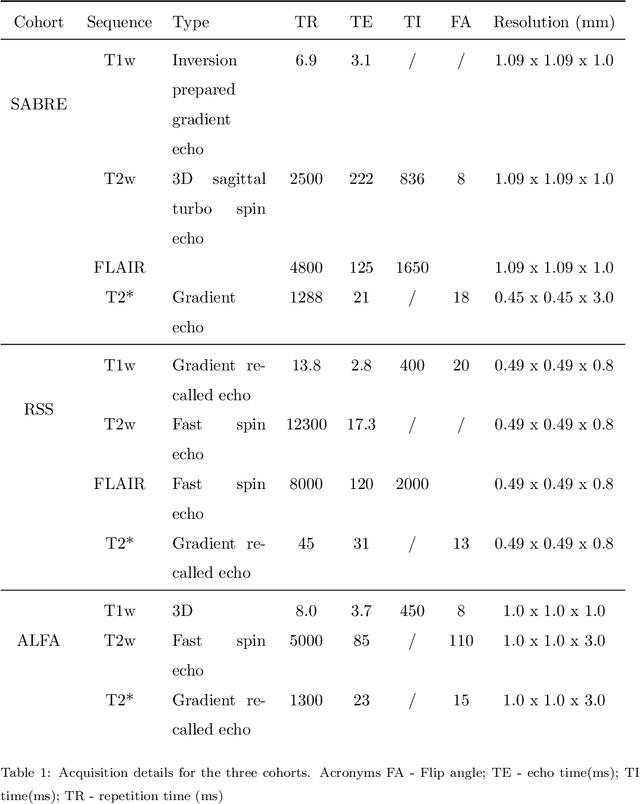
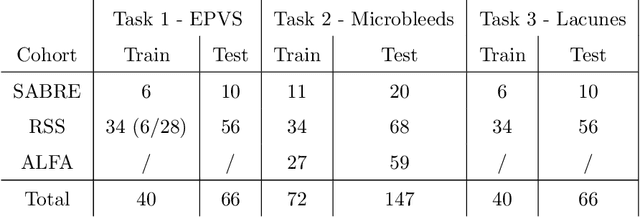
Abstract:Imaging markers of cerebral small vessel disease provide valuable information on brain health, but their manual assessment is time-consuming and hampered by substantial intra- and interrater variability. Automated rating may benefit biomedical research, as well as clinical assessment, but diagnostic reliability of existing algorithms is unknown. Here, we present the results of the \textit{VAscular Lesions DetectiOn and Segmentation} (\textit{Where is VALDO?}) challenge that was run as a satellite event at the international conference on Medical Image Computing and Computer Aided Intervention (MICCAI) 2021. This challenge aimed to promote the development of methods for automated detection and segmentation of small and sparse imaging markers of cerebral small vessel disease, namely enlarged perivascular spaces (EPVS) (Task 1), cerebral microbleeds (Task 2) and lacunes of presumed vascular origin (Task 3) while leveraging weak and noisy labels. Overall, 12 teams participated in the challenge proposing solutions for one or more tasks (4 for Task 1 - EPVS, 9 for Task 2 - Microbleeds and 6 for Task 3 - Lacunes). Multi-cohort data was used in both training and evaluation. Results showed a large variability in performance both across teams and across tasks, with promising results notably for Task 1 - EPVS and Task 2 - Microbleeds and not practically useful results yet for Task 3 - Lacunes. It also highlighted the performance inconsistency across cases that may deter use at an individual level, while still proving useful at a population level.
Let's agree to disagree: learning highly debatable multirater labelling
Sep 04, 2019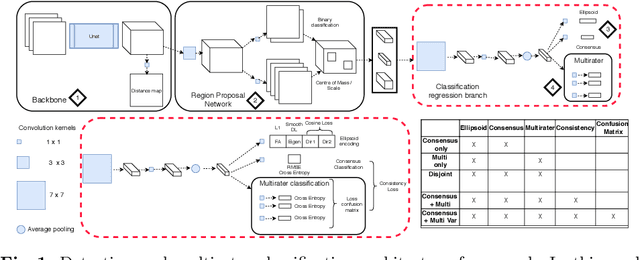
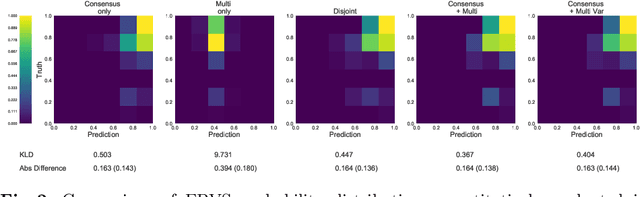
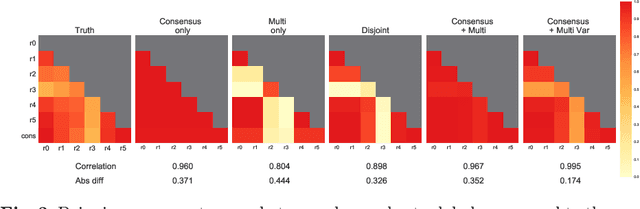
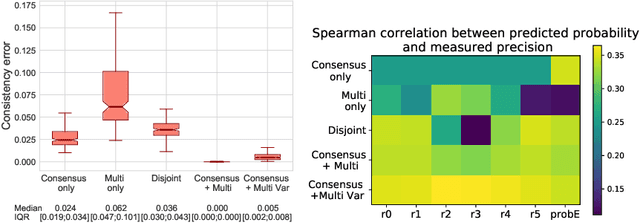
Abstract:Classification and differentiation of small pathological objects may greatly vary among human raters due to differences in training, expertise and their consistency over time. In a radiological setting, objects commonly have high within-class appearance variability whilst sharing certain characteristics across different classes, making their distinction even more difficult. As an example, markers of cerebral small vessel disease, such as enlarged perivascular spaces (EPVS) and lacunes, can be very varied in their appearance while exhibiting high inter-class similarity, making this task highly challenging for human raters. In this work, we investigate joint models of individual rater behaviour and multirater consensus in a deep learning setting, and apply it to a brain lesion object-detection task. Results show that jointly modelling both individual and consensus estimates leads to significant improvements in performance when compared to directly predicting consensus labels, while also allowing the characterization of human-rater consistency.
3D multirater RCNN for multimodal multiclass detection and characterisation of extremely small objects
Dec 21, 2018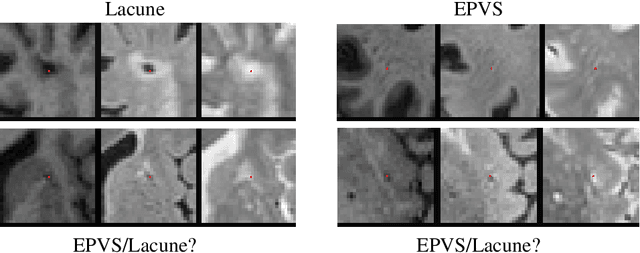
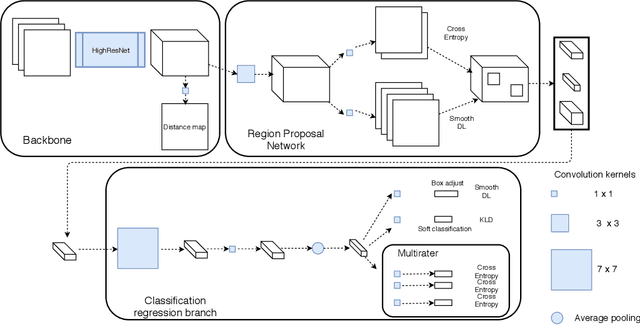
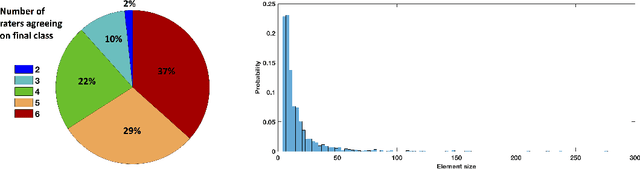
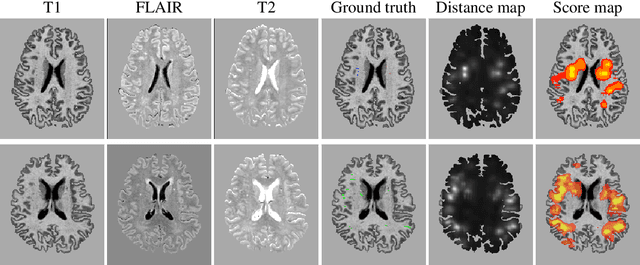
Abstract:Extremely small objects (ESO) have become observable on clinical routine magnetic resonance imaging acquisitions, thanks to a reduction in acquisition time at higher resolution. Despite their small size (usually $<$10 voxels per object for an image of more than $10^6$ voxels), these markers reflect tissue damage and need to be accounted for to investigate the complete phenotype of complex pathological pathways. In addition to their very small size, variability in shape and appearance leads to high labelling variability across human raters, resulting in a very noisy gold standard. Such objects are notably present in the context of cerebral small vessel disease where enlarged perivascular spaces and lacunes, commonly observed in the ageing population, are thought to be associated with acceleration of cognitive decline and risk of dementia onset. In this work, we redesign the RCNN model to scale to 3D data, and to jointly detect and characterise these important markers of age-related neurovascular changes. We also propose training strategies enforcing the detection of extremely small objects, ensuring a tractable and stable training process.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge